nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
Alternate solvent for grignard
Hi
i was looking for some solvent were grignard can be carried
i saw Ether, tetrahydrofuran,
and i was wondering if pyridin or 1-methylpyrrolidine could be used
i saw 1 mention of 1-methylpyrrolidine
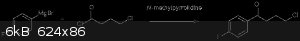
[Edited on 12-12-2015 by nelsonB]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Pyridine and N-methylpyrolidine are probably gunna give junk, and will be a pain to remove from your desired product.
Another ether like 1,4-dioxane or dimethoxyethane may work, but they are not ideal. Ethers are required to coordinate Mg complex, and Et2O is going to
be your best bet. It is relatively cheap, simple enough to dry, and very easy to remove.
Is ether difficult to acquire for you?
|
|
|
nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
i just had a tought that during the grignard reaction *Phenyl* chloride might react with Pyridine to form a quaternary amine,
also its not because its hard to aquire,
its because the some reagent are little soluble in ether,
such acetamine
[Edited on 13-12-2015 by nelsonB]
[Edited on 13-12-2015 by nelsonB]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
so you want to make an amphetamine using the Grignard route? It's not a productive way. I can see why you thought of it, Grignards are easy and fun to
watch. Have a bowl of ice water ready.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
I don't know were you see that reaction could give that product but if we follow that chart its should give acetophenone.
its pretty much like the synthesis above but acetamide is simpler to made than acetic acid chloride
the amine is not keep after the reaction
its like the chlorine on the acetic acid chloride
also i writen the wrong name of chemical product
confusing Phenyl chloride with benzyl chloride
that they are not the same product.
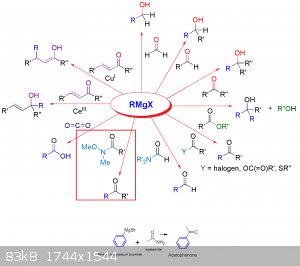
[Edited on 13-12-2015 by nelsonB]
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
If you look at that image you linked more closely you will notice that there is one Me group and one MeO group attached to the nitrogen, so I'm not
sure acetamide will react the way you want it to. Also your desired product is a ketone... can you see why that might be problematic in a grignard
reaction?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Primary and secondary amides are acidic enough to destroy Grignard reagents (the Grignard reagent will act as a base instead of a nucleophile, and
deprotonate it; see https://en.wikipedia.org/wiki/Nucleophilic_acyl_substitution...). You'll need to use acetonitrile, or a tertiary acetamide, instead of acetamide.
[Edited on 12-13-2015 by Cheddite Cheese]
|
|
|
nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
yeah NitreRat i figured that but i think its need to look a closer look to the reation metanism there might be an intermediate in this,
since we can see in the chart that its give an ketone
|
|
|