kamal
Harmless

Posts: 33
Registered: 14-9-2005
Member Is Offline
Mood: always happy
|
|
Acyl chloride on TLC
I am preparing 4-Phenyl butyryl chloride from 4-Phenylbutyric acid & thionyl chloride. I tried different processes like taking reaction in benzene
at reflux, with SOCl2 as solvent (in all cases SOCl2 in 4-5 mol. excess). But while checking for reaction completion thro TLC, the acid shows big spot
even after a long run time.....
Here, I'm not sure, but is it possible that the acyl chloride gets hydrolyze on tlc plate giving back the acid..? Can the Acyl chloride be detected by
tlc ?
Awaiting your suggetions....
|
|
|
Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
Not too long ago I tested the purity of some benzoyl chloride, and it worked fine... Using iodine for the visualization.
Of course it's impossible to tell whether the acyl halide is hydrolyzed in the above test, without a reference Rf value, or a reference test with pure
benzoic acid... However I assume it hasn't. Maybe that was foolish of me...
Your product should be visible with most visualizating systems - UV light, iodine, KMnO4/base, vanillin etc.
Though... You might want to switch visualizating agent anyway? Just for the sake of certainty?
If that doesn't work then perhaps you should try a two-dimensional TLC development... The procedure is explained Vogel's I think.
If that doesn't give any usable results, then I'm out of ideas.
Good luck, and keep us updated!
- Furch
[Edited on 7-1-2007 by Furch]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
What's the mobile phase?
Anyway, the silica gel coating on TLC plates is very hygroscopic so hydrolysis back to the starting material is possible unless you very carefully
dried the plates first.
IIRC the aliphatic acid halides hydrolyse quicker than aromatic so you mighht be able to TLC benzoyl chloride but not phenyl butyryl chloride.
|
|
|
kamal
Harmless

Posts: 33
Registered: 14-9-2005
Member Is Offline
Mood: always happy
|
|
unionised....
So, according to you, I can assume that the reaction is going on or may be completed but I can not predict/check its completion.....
Are you sure about this hydrolysis....?
BTW, the solvent system I use is 30% EtOAc in n-Hexane.
|
|
|
Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
Aye, I would have to agree with that statement... Concerning the reactivity of aromatic vs. aliphatic acid chlorides.
However, if I was to synthesize this compound, I would probably determine the purity by observing its boiling point during the second distillation in
the clean up, rather than TLC, since there are obvious doubts as to the functionality of TLC concerning this particular substance.
I don't have MERCK at hand, but I'm sure it's in there somewhere.
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
If there is a functional derivative like an amide you could make a m.p. determination directly and easily
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Do you have access to an LCMS machine or similar? Then you could at least see if there is a product with the right mass. Although the one in my lab
uses aqueous acetonitrile as the solvent, so you might get the problem of hydrolysis again...
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I have recently made lots of cinnamoyl chloride. To determine the extent of reaction a tiny amount was weighed and to this was added a weighed amount
of dry methanol. The methyl ester was then quantified using HPLC (C18, MeOH:H2O gradient, UV at 254, 280 and 300nm). The resulting mix could also be
resolved on Si(OH)n with something like EtOac:hexanes and visualized conveniently with I2 in Si(OH)x (in a jar) or by looking for UV quenching on the
plates.
If done correctly, the yields for this Chemistry are usually quite high, 95% for example, with cinnamoyl chloride.
I also have found that cinnamoyl chloride (and likely your acyl chloride) will penetrate both latex and nitrile gloves. If you notice a red brown spot
on your gloves, remove them immediately and check for a "wet" area where the spot was. If you have one, the spot will test strongly acidic to pH
paper. I found that scrubbing twice with granular NaCO3 (moistened with water) and washing thoroughly with water did the trick. The skin will fall off
some days later.
Be careful and good luck,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Is there a reason for using solvent (benzene)? Simply mix the acid with excess of SOCl2, allow to react for 1-2 hours and remove SOCl2 in vacuo. The
reaction should proceed in quantitative yield. It is impossible to have a significant quantity of starting material left if you preform the reaction
analogus as this:
(− -(1S,4R)-Camphanoyl chloride. A 500-mL, three-necked, round-bottomed flask,
equipped for magnetic stirring and protected from moisture by a reflux condenser topped by a CaCl2 drying tube, is charged with 200 mL of thionyl
chloride using a graduated cylinder. (− -(1S,4R)-Camphanoyl chloride. A 500-mL, three-necked, round-bottomed flask,
equipped for magnetic stirring and protected from moisture by a reflux condenser topped by a CaCl2 drying tube, is charged with 200 mL of thionyl
chloride using a graduated cylinder. (− -(1S,4R)-Camphanic acid (63.8 g, 0.322
mol) is added in portions using a powder funnel over 30 min, and the reaction mixture is heated under reflux for 3 hr, then allowed to cool to room
temperature. Excess thionyl chloride is removed by rotary evaporation to afford a solid that is freed of any residual thionyl chloride by the addition
of toluene (500 mL) and subsequent evaporation under reduced pressure (repeated three times). The resulting solids are dried under high vacuum (Note
5) to afford 69 g of (− -(1S,4R)-Camphanic acid (63.8 g, 0.322
mol) is added in portions using a powder funnel over 30 min, and the reaction mixture is heated under reflux for 3 hr, then allowed to cool to room
temperature. Excess thionyl chloride is removed by rotary evaporation to afford a solid that is freed of any residual thionyl chloride by the addition
of toluene (500 mL) and subsequent evaporation under reduced pressure (repeated three times). The resulting solids are dried under high vacuum (Note
5) to afford 69 g of (− -(1S,4R)-camphanoyl chloride (99%) as an off-white
solid, mp 69–71°C. -(1S,4R)-camphanoyl chloride (99%) as an off-white
solid, mp 69–71°C.
[Edited on 8-1-2007 by Sandmeyer]
[Edited on 8-1-2007 by Sandmeyer]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Is there a reason for using solvent (benzene)? Simply mix the acid with excess of SOCl2, "
I think he tried that.
"I tried different processes like ... with SOCl2 as solvent "
I wonder just how many people who have ready access to LCMS use TLC but, anyway, you'd be stuck with normal phase LC which never seems to work so well
when I do it.
Adding methanol or some such and assaying the ester seems like a good option if you really must use TLC but I have to agree that the boiling point is
certainly the easy way to check that you have a pure product that isn't the same as the starting materials. (OK you might get really unlucky with an
azeotrope but that's life)
[Edited on 8-1-2007 by unionised]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
AH fuck! Whole post typed when "num lock" is hit accidentally, then add a degree symbol and whammo, back in explorer and bye-bye post . .
Anyway, apologies for my profanity, and once again:
I like to use TLC because it is frequently faster (10 mins. vs ~30min for the reverse phase LC separation gradient I made) and multiple spots can be
done at once (which is great if you are sans autosampler). Unfortunately, the TLC does not provide comparable separation (the cinnamic acid does not
move much which leads to streaking). Just learn how to pull capillaries from cheap pasteur pipettes!
On Si(OH)n, cinnamic acid methyl ester is effectively non polar (OK, medium with exceptions) and hexane with a tweak of a similar solvent, like EtOAc
does the trick.
As for a solvent, I too have tried the SOCl2 solvent route as well. This is great if the substrate and product are both liquid, or can be easily
distilled. It is not-so-great if the acid and the acyl chloride have relatively high boiling points (where distillation in-vacuo leads to the
formation of "tar"...). In the case of cinnamic acid, I have found a solvent, particularly toluene, especially helpful.
1) The solvent neatly dissolves the cinnamic acid prior to SOCl2 addition which increases the rate of mass transfer (faster, smoother rxn., higher
yield because of less tar--which is formed by very high viscosities and localized superheating).
2) The boiling point of toluene is higher than SOCl2, but low enough to prevent excessive tar formation. It is a good chaser, distilling without
bringing the product with it. I have found that up to 99% of the toluene can be easily removed by bringing the *pot* temp. to 65°C (whew, num lock
on!), and carefully using the vacuum pump to pull over the solvent until the pot reaches 55°C, repeat as needed and presto, the pot contains 98%
cinnamoyl chloride with a yield of ~95% m/m). At this pot temp and a decent vacuum (estd. 20mmHg, transient), this acyl chloride, at least, does not
azeotrope (it *does* at STP and 110° a the *head*--this is a mess, the entire apparatus ends up coated with cinnamic acid on contact with moist
air--acetone takes it right off).
I did the unlucky part so you don't have to!
Tomorrow, I'll get a screen dump of the HPLC chromatogram and a photo of the TLC plate for reference. Come to think of it, I'll document the next
batch (I think it's a good general lab exercise).
Best of luck and Cheers!
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
It has nothing to do with if substrate/product is solid or liquid, nor boiling points, you just remove SOCl2 under vacum, or is it too simple maybe?
[Edited on 9-1-2007 by Sandmeyer]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
If you are going to do that you must use some solvent anyway to chase out any SOCl2 traces. If you do not (and you plan to use, say, pyridine as your
proton sink) a severely dehydrating (with exothermia) reagent complex is formed. See pyridine:SOCl2 (Fieser, vol. 1). This frequently leads to
instantaneous formation of tar.
If you have had great results this way, more power to you (I'd be happy to hear a full synthetic account to point out the points where what you are
doing is different). I simply have included here, a route that has worked well for me and why I think that this is the case.
Oh, and watch the pot temp not the head temp or azeotrope you will receive!
Take care all,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sandmeyer
Simply mix the acid with excess of SOCl2, allow to react for 1-2 hours and remove SOCl2 in vacuo. The reaction should proceed in quantitative yield.
|
Actually some carboxylic acid just don't react efficiently with SOCl2 or do so slowly (I have no idea which acids in particular cause such problems
and why). In such cases a couple of drops of DMF or pyridine added to the refluxing mixture of the acid in SOCl2 catalyses the reaction (by means
unknown to me). At least this is what is written under the acid chloride preparation entry in Organikum (the book - not the forum member).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Try dry alumina plates. Silica (plates and chromatography powder alike) contains up to 9% water IIRC.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hello!
Ah yes, the DMF trick (now remember mention of this in Fieser). Also in Fieser (vol I) is the SOCl2:pyridine complex which when present where not
intended leads to dehydration and carbonized tar (at least with things like citric acid).
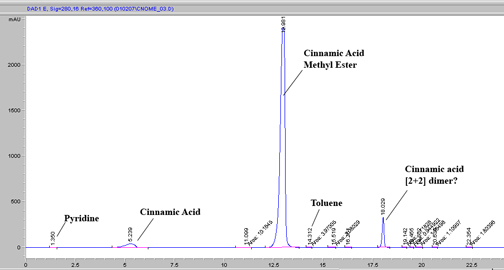
I ran the TLC of cinnamic acid and cinnamic acid methyl ester (from cinnamoyl chloride in dry methanol) today. It worked very well on desicated
(relative humidity in dry box was ~40% p/p*) Si(OH)x with a toluene:EtOAc eluent made at 1:3.33; RF CAOMe: 0.75. I have photos of this, but left the
camera at the lab, so I will edit and post them tomorrow.
Alumina is usually applied according to the Brockman activity (and also, pH, which can also be adjusted), which is a function of the amount of water
contained therein (it is frequently hydrated by the user for specific purposes). When used, Al2O3 adsorbent is almost always given with the
corresponding Brockman activity when submitted for publication (otherwise, duplication would be extremely difficult).
I have noticed that bone-dry Si(OH)x behaves strangely, as if k (capacity) is very small which leads either to lack of mobility or more likely, severe
streaking. Has anyone else noticed this? I suspect that the granules require a certain amount of moisture to swell sufficently for k to be useful.
Likewise, operating C18 in pure water is a no go as the hydrophobic "tails" lie down greatly diminishing the amount of sorbent surface available.
The chromatogram of my reaction mixture is given below with a MeOH:H2O (1mL/min) gradient on C18 (250mm X 4mm, 5u) and extracted wavelength of 280nm.
Cheers!
[Edited on 9-1-2007 by Ozone]
[Edited on 9-1-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
kamal
Harmless

Posts: 33
Registered: 14-9-2005
Member Is Offline
Mood: always happy
|
|
Ah...!
Great suggetions & discussion from all you friends.....
I tried one suggetion of making the ethyl ester from the acyl chloride compound which I made by adding the acyl chloride into absolute ethanol &
kept for some time. Then I took the tlc which did not show any spot of my parent acid....! 
So, ultimately it seems that this acyl chloride gets hydrolyzed on the tlc plate giving the parent acid. And I have not to be worried for my
conversion of acid to acyl chloride...!!  It is as easy as it looks. It is as easy as it looks.
More new suggetions are welcome.
Thanks to all of you.....
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
BTW, if you're spotting from DMF, forget about getting decent TLCs. It'll cause huge streaks.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
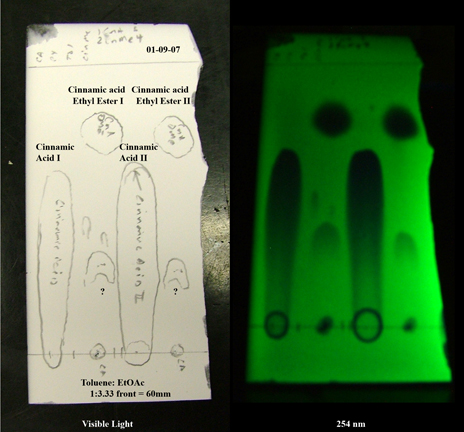
That is absolutely true--DMF co-solves just about everything. Result--streaks!
For example, in the following TLC (as promised) the cinnamic acid streaks (also, as promised) because the eluent is optimized for the much less polar
methyl ester. A drop or two of AcOH of TFA will tighten it up at the expense of limited mobility of less polar species.
Sorry about the plate edges, but I was in a hurry and the glass cutter rebelled quite furiously.
Cheers,
O3
[Edited on 11-1-2007 by Ozone]
[Edited on 11-1-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Oh, and here is a picture of cinnamoyl chloride product synthesized left, in SOCl2 solvent and right, in toluene.
Tar sucks!
regards,
O3

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
You might want to distill or sublime (possible?) that one on the left...
[Edited on 11-1-2007 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I think that the one on the left is defunct (that is, I'll dispose of it). The newer improved synthesis (product on the right) scales well and
continues to produce high yields and is of sufficient purity to use as-is. Besides, distilling the stuff is a pain! you need to wrap the whole
apparatus with heating tape to prevent the stuff from solidifying in the condenser, etc.
I don't think it sublimes; when heated it seems to liquify then if heated more...makes more tar. The nasty one also has a lower melting point than the
one on the right. The white one melts at a temperature that is very close to the literature value.
I live, I learn,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|