DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Preparation of 4-chlorobutyl acetate
I am attempting to replicate one of the pathways to 4-chlorobutanal dimethyl acetal, as per "Synthesis of tryptamines by the Fischer method using
synthetic precursors and latent forms of aminobutanal" (Bidylo, Yurovskaya, 2008).
I have had reasonable success so far. The first step is the production of 4-chlorobutanol. This is prepared with reacting tetrahydrofuran (THF) with
acetyl chloride (AcCl) with a zinc chloride catalyst. I am basically using the process in the paper "The reaction of tetrahydrofuran and
2,5-dimethyltetrahydrofuran with acyl halides" (Cloke, Pilgrim, 1930).
41.24g of THF (0.573 mol) is placed in a 250ml 2-necked flask with 15mg of anyhydrous ZnCl2, fitted with a reflux condenser and a pressure equalizing
funnel.
53.62g of AcCl is placed in the funnel and the apparatus flushed with nitrogen. The contents are protected from atmosphere using a septum made from a
nitrile glove.
The AcCl is added dropwise with magnetic stirring over the course over approximately 0.5hr. At the completion of the addition, the mixture is refluxed
for a further hour. I have an oil bath heater which was set to 80C for the reflux.
The completion of the reaction can be seen when all the AcCl is consumed and no further reflux occurs.
The reaction mixture is then briefly heated at 100C to remove any unreacted THF and AcCl. The apparatus is then configured for distillation.
I collected the fraction coming over at a temperature of 100C and pressure of 0.05atm, with a total amount of 59.14g recovered. 8.1g of a yellowish
viscous liquid remained in the flask.
Density 1.08g/ml at 20C.
59.14g of 4-chlorobutyl acetate is 0.39mol, a yield of 68%. Cloke reported a yield of 76%.
I performed this reaction in a fume hood given the corrosive vapours from the AcCl, and also ensured good scrubbing of the vapours between my
distillation set up and my pump.
The next step is the transesterification of the acetate using methanol. This procedure is also in the Cloke paper, I'll report on that separately.
Bidylo also provides references to preparation of diol using acetyl chloride with tetrahydrofurfuryl alcohol (THFA) with a similar process (I
believe). Unfortunately, I cannot access the references:
Paul, R C.R. Acad. Sci. Paris 211:645 (1940); 215:303 (1942)
Paul, R Bull. Soc. Chim. Fr., 911 (1941)
Paul, R & Tchelitichef, S Bull. Soc. Chim. Fr., 197 (1948)
If anyone can obtain these papers, I would be very grateful.
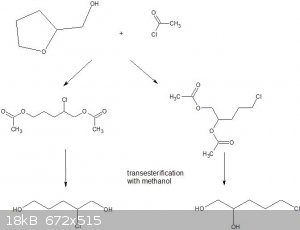
I have attempted the reaction with THFA, and obtained *something* with a boiling point around 110C at 0.05atm.
I am speculating that the reaction can go a couple of different ways;the figure below also shows the final desired vicinal diol together with a
1,5-diol. Comments welcome!
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Nicely done! I'll see if I can add those references when I have access to an actual computer and not just a phone.
How are you planning on performing the oxidation?
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
I am thinking TEMPO for the oxidation. There are several other approaches mentioned in the Bidylo paper, but they all involve relative nasties like
PCC, or Swern reagents. TEMPO appeals because it works at low temperatures, and there is a comment that the 4-chlorobutanal is unstable even at fairly
low temperature. Also, only about 1mol% is required.
I'm going to be following the general guidelines of "A general synthetic method for the oxidation of primary alcohols to aldehydes..." (Anelli,
Montanari, Quici, orgsyn, 1990)
The co-reagents, potassium bromide and sodium hypochlorite, are cheap and readily available.
I am a bit worried about the water solubility of the 4-chlorobutanal - I can't find much data on it, so the workup to bring the 4-chlorobutanal into
the organic phase (according to the above paper) may not turn out so well.
I will then move directly to the acetalization of the aldehyde without attempting to separate it from the solvent.
One of the reasons I was looking at the tetrahydrofufuryl alcohol and the resulting vicinal diol was that I had found a paper with an oxidation
process that took vicinal diols directly to aldehydes using Ca(OCl)2, ie very cheap oxidants.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
If you can get your hands on some, perruthenate works very well to oxidize alcohols to aldehydes with minimal byproducts. All you need is an amine
oxide, molecular sieves to catch water produced in the reaction, and a few mole percent of a perruthenate salt and you're good to go.
Although I'm guessing this annoying oxidation is the reason people consider doing Grignards to get to this substrate in the first place... 
|
|
|
bromisovalum
Harmless

Posts: 3
Registered: 18-12-2017
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Paul, R C.R. Acad. Sci. Paris 211:645 (1940); 215:303 (1942)
|
I've found these two papers. There's some interesting tidbits in CR 215:303 (1942), they say that the mixture of 5-chloro-1,2-pentanediol and
2-chloro-1,5-pentanediol, obtained by the action of acetyl chloride on tetrahydrofurfuryl alcohol, was oxidised by lead(IV)acetate in diethyl ether to
4-chlorobutanal. They add that Pb(OAc)4 can be substituted by sodium periodate, but without significant advantages.
4-chlorobutanal is described as a colourless liquid with a penetrating odour, but more agreeable than butanal, bp. 51-53°C (13 mmHg), prone to
polymerisation especially when heated.
Tetrahydrofurfuryl alcohol is produced by catalytic hydrogenation of furfural from biomass, I've seen it touted as a "green" industrial solvent.
Attachment: php0kqUJx (367kB)
This file has been downloaded 283 times
Attachment: phpQeJujP (430kB)
This file has been downloaded 262 times
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Awesome! I had forgotten about those papers. Many thanks for digging them up. For some reason, they had lost the .pdf file extension, but fixed that
OK.
Now of course all I need is to learn French...time to try some translation tools.
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
To follow up, I have put the papers through Google translate and tidied up the English, enough at least to understand what is going on. I haven't
transcribed the tables that were in the source docs. I have put both documents into the one translation.
For my purposes, it appears the oxidation to the aldehyde can take place directly on the mix of alcohols/diols resulting from the action of acetyl
chloride on tetrahydrofurfural alcohol. And it seems the products from that reaction is as I surmised.
Attachment: Pentanetriol.pdf (201kB)
This file has been downloaded 313 times
|
|
|