CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Purity of battery sulfuric acid
Where I live, battery acid can be cheaply bought at any automotive store. It is crystal clear, but I was wondering if it contains any major
impurities. I think it should be quite pure so that the battery runs properly. The only impurity that it could definitely have is lead sulfate, but
that has a solubility of 1.2 ppm at that concentration, so even a saturated solution won't have much. Does anybody know if battery acid basically
equates to diluted reagent grade sulfuric acid?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Battery electrolyte, new in the container from an auto parts store, never installed in a battery is quite useable, but is only 30% concentration.
Google on boiling battery electrolyte, it can be concentrated to 80% +.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Bert  | Battery electrolyte, new in the container from an auto parts store, never installed in a battery is quite useable, but is only 30% concentration.
Google on boiling battery electrolyte, it can be concentrated to 80% +. |
I know. I concentrated it myself to 90.4% (determined through titration) without too much effort. Buy my main concern were the impurities and their
nature as they could affect some reactions. Anyway, I think I will do like Robert Bruce Thompson and neutralize it with base and then weigh the
residue of evaporation and see if it weighs more than the calculated mass of sodium sulfate.
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Battery electrolyte in its virgin form is quite pure sulfuric acid for obvious reasons and very much usable for any amateur experimentation that I can
think of.
I would be more cautious about contaminants that can enter the acid during the concentration (boiling off water) phase. If the vessel that the process
is performed in is not resistant enough against the effects of boiling hot acid then there is a chance of contaminating the product. Otherwise I would
not be too worried about any unwanted congeners.
Exact science is a figment of imagination.......
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
I'd think it's quite pure, especially when you consider that you're meant to top up old lead acid batteries with distilled water rather than tap
water, which would imply impurities are problematic in lead-acid batteries.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
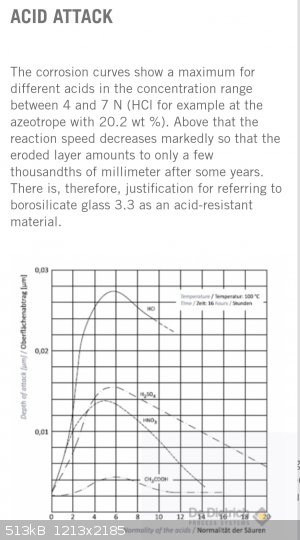
The starting 30% battery electrolyte is close to the most corrosive concentration of boiling Sulfuric acid towards borrosilicate glass (25%).
Boiled down H2SO4 done in glass WILL take up a small ammount of material from Pyrex, particularly the alkali metals, the small % of Sodium in the
glass definitely can get leached out measurably.
This never affected any process I used such acid for- Toluene, glycerin, cellulose, erythritol, pentaerythritol, mannitol, dinitrochlorobenzene,
salicylates, resorcinol... None of them seemed to mind a few Na atoms in the mix.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Bert  | The starting 30% battery electrolyte is close to the most corrosive concentration of boiling Sulfuric acid towards borrosilicate glass (25%).
Boiled down H2SO4 done in glass WILL take up a small ammount of material from Pyrex, particularly the alkali metals, the small % of Sodium in the
glass definitely can get leached out measurably.
This never affected any process I used such acid for- Toluene, glycerin, cellulose, erythritol, pentaerythritol, mannitol, dinitrochlorobenzene,
salicylates, resorcinol... None of them seemed to mind a few Na atoms in the mix.
|
Of course I don't mind that little sodium. It is way beyond the capabilities of measurement I have anyway, but interesting information nonetheless.
[Edited on 15-3-2018 by CobaltChloride]
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
@CobaltChloride Battery acid should only have sulfuric acid and water, though in a few bottles I also found a bit of white precipitate, which could
have been either plastic waste or lead sulfate. After I simply decanted off the precipitate, I reacted the acid with ammonium bicarbonate and heated
the ammonium sulfate in a test tube until it completely decomposed. No residue was left in the tube, meaning there aren't any metallic salts in
battery acid.
Also, if you need concentrated acid, look for a Czech brand, CLEAMEN 420 (Leroy Merlin and Praktiker sell it). It's almost crystal clear (some bottles
are slightly dark cloudy, though not all), and it is around 93-95% sulfuric acid. I too tested it in the same way like the battery acid, and there was
no residue left in the tube. I assume the slight dark coloring is some organic residues.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
battery acid must be very pure or it will poison the cell and cause large self discharge rates and more issue with charge absorption (I live off flood
lead acid cells!)
So if it is virgin from reputable store it will be very very pure just shy of acs grade is any thing!
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Mabus  | @CobaltChloride Battery acid should only have sulfuric acid and water, though in a few bottles I also found a bit of white precipitate, which could
have been either plastic waste or lead sulfate. After I simply decanted off the precipitate, I reacted the acid with ammonium bicarbonate and heated
the ammonium sulfate in a test tube until it completely decomposed. No residue was left in the tube, meaning there aren't any metallic salts in
battery acid.
Also, if you need concentrated acid, look for a Czech brand, CLEAMEN 420 (Leroy Merlin and Praktiker sell it). It's almost crystal clear (some bottles
are slightly dark cloudy, though not all), and it is around 93-95% sulfuric acid. I too tested it in the same way like the battery acid, and there was
no residue left in the tube. I assume the slight dark coloring is some organic residues. |
Thank you for the information about cleamen 420. I've seen it in stores, but I bought something else which wasn't as good.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Battery acid contains about 30 to 40% H2SO4
And it is pure . If you are thinking it contains lead then it is not since lead sulphate is insoluble.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by Akhil jain  | Battery acid contains about 30 to 40% H2SO4
And it is pure . If you are thinking it contains lead then it is not since lead sulphate is insoluble. |
It has a limited solubility in sulfuric acid though. Enough so that a saturated solution could make chemicals whose impurity is detectable.
|
|
|