| Pages:
1
2
3
4
5 |
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by JJay  | | I will be actively working to synthesize oxalyl chloride next week. I'll check back, and if someone has actually beaten me to the punch then I'll
award the prize, but I have an advantage: under the rules, as the contest sponsor, I am permitted to use phosphorus compounds. So if you have a
winning entry, now is the time to submit it. |
Yeah, I don't see anything about that in the OP. Lame.
But if you want to race, I could probably whip up some PCl5 next week...
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | Quote: Originally posted by JJay  | | I will be actively working to synthesize oxalyl chloride next week. I'll check back, and if someone has actually beaten me to the punch then I'll
award the prize, but I have an advantage: under the rules, as the contest sponsor, I am permitted to use phosphorus compounds. So if you have a
winning entry, now is the time to submit it. |
Yeah, I don't see anything about that in the OP. Lame.
But if you want to race, I could probably whip up some PCl5 next week... |
Read the fine print.
I have a few things on my plate. You can probably do it faster, but the prize is for a non-phosphorus synth. Or if you want, you can take charge of
the challenge, but then you have to award the prize. Up to you. I keep my word.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Ok, I have one more stupid idea.
Suppose your starting materials are ethyl bromide, thiocyanate, DMSO, and acetyl chloride.
The first two give ethyl thiocyanate.
The second two give chloromethyl methyl sulfide:
https://pubs.acs.org/doi/abs/10.1021/ja01608a016?journalCode...
It should be possible to perform a Barbier-like reaction between these two to get ethyl thiomethylthioacetate. Chloromethyl methyl sulfide is likely
to be a noxious chemical, but it should be less volatile than Me2S.
Oxidation with SeO2 should give oxalyl methyl-ethyl dithioester, which then might interact with SO2Cl2 to give oxalyl chloride.
[Edited on 15-12-2018 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by JJay  | Quote: Originally posted by Texium (zts16)  | Quote: Originally posted by JJay  | | I will be actively working to synthesize oxalyl chloride next week. I'll check back, and if someone has actually beaten me to the punch then I'll
award the prize, but I have an advantage: under the rules, as the contest sponsor, I am permitted to use phosphorus compounds. So if you have a
winning entry, now is the time to submit it. |
Yeah, I don't see anything about that in the OP. Lame.
But if you want to race, I could probably whip up some PCl5 next week... |
Read the fine print.
I have a few things on my plate. You can probably do it faster, but the prize is for a non-phosphorus synth. Or if you want, you can take charge of
the challenge, but then you have to award the prize. Up to you. I keep my word. |
I see no fine print to read.
I even searched for "phosphorus" on the first page of this thread and it was only mentioned twice- once in the OP where you said "You must
synthesize oxalyl chloride without using any phosphorus compounds" and a second time in the quoted post.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by JJay  |
5. I reserve the right to close the competition before any submissions are received by producing oxalyl chloride in my own lab.
|
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Don't know what has you so upset Jjay. This seems like an overreaction.
I hope you reconsider. You will be missed.
|
|
|
andy1988
Hazard to Others
  
Posts: 135
Registered: 11-2-2018
Location: NW Americus ([i]in re[/i] Amerigo Vespucci)
Member Is Offline
Mood: No Mood
|
|
I'd tried to reply to this thread earlier but it was closed, j_sum1 suggested I post it here.
I thoroughly enjoy your contributions JJay, and style of writing/debate e.g. [1][2].
Reminds me of reading through early 1900s scientific proceedings, authors have interesting argumentation and style (in this page, lavish praise for Dr. Cook) (somewhere in a different volume/book an author when
challenged said if he was shown wrong, to treat his writing as detritus, reminded me of this forum).
To illuminate an example of what I believe JJay was referring to as blatant self-promotion, see my posting here and later that page Melgar here. I chuckled extensively but my intention was never to maintain such a script, but after writing it to try to pawn it off on someone else to
maintain and run, such as streety. I really am a "lazy" programmer, I hate to do tasks outside of what I'm principally focused on and I pawn such
tasks/projects off for others to do at work. I hardly have the focus/energy to do everything I do want to do.
My motivation in things is to learn, share knowledge, and teach; I don't care for praise or recognition. It brings me joy to bring others success! On
this topic I am thankful for the efforts Melgar and streety had made (and countless others for their efforts on different subjects). Though I guess
there are other things which bothered JJay in this matter, e.g. the suggestion of compensation for tasks, running contrary to the spirit of this forum
(I'm sure this could be articulated better, but I have shopping to do before this night is through!).
[Edited on 16-12-2018 by andy1988]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
This challenge is still on.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
2018 atara: lots of complicated ideas
2020 atara: fuck it just hit glyoxal with some chlorine and see what happens
(be careful!)
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I have thought about that, but hoping that glyoxal will be formed in situ from ethylene glycol and chlorine. Unfortunately it rather didn't work.
I couldn't get glyoxal enywhere and synthesising it seems like something impossible.
Good luck in getting some.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Glyoxal can be prepared by the action of nitric acid on ethanol and purified by the bisulfite adduct, according to this 1850s paper: https://books.google.com/books?id=S1AwAAAAIAAJ&pg=PA39#v...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by mackolol  | | hoping that glyoxal will be formed in situ from ethylene glycol and chlorine. Unfortunately it rather didn't work. |
I don't think this can work because the intermediate glycolaldehyde immediately dimerizes
[Edited on 24-6-2020 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The dimerization would be reversible, wouldn't it?
[Edited on 24-6-2020 by JJay]
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I have tried a few syntheses of glyoxal, one of it being Polish patent oxidation of ethylene glycol by blowing air into boiling mix of EG/copper
acetate which was supposed to work as a catalyst. I don't know what was I expecting, but you can easily figure out that it didn't work for me.
I know how shitty aldehydes can be in terms of purification, and glyoxal, being the simplest dialdehyde is a nightmare to work with. I gave up on
this.
And you know, I can buy glyoxal to be honest but besides it being pretty expensive, it is sold as a 40% solution in water... I don't know if isolating
it from water solution and then dehydrating it can be done in reasonable yield and quantity.
[Edited on 24-6-2020 by mackolol]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'd think that oxidation of ethylene glycol with PCC or chromium VI oxide peroxide etherate would work. I don't think the yields would be spectacular
with chromium oxidizers.
TEMPO oxidation with something simple like bleach would be more environmentally friendly and perhaps higher-yielding.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Has anyone considered chlorinating ethylene carbonate and decomposing the tetrachloroethylene carbonate by some means? A cursory look gave me a a hit.
And I think ethylene carbonate can be made from urea and ethylene glycol quite easily, even without catalysts such as described here. I wouldn't be surprised if there is a thread on here about making dimethyl carbonate via a similar sequence.
Edit: ahh I see I broke rule 3 of OP, but why would you have that rule? It's not as if the product is any less nasty aside from being a liquid instead
of a gas.
[Edited on 24-6-2020 by Sigmatropic]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
But "being a liquid instead of a gas" is the whole reason we use oxalyl chloride instead of phosgene in the first place!
If I were trying to make glyoxal from first principles, I'd try nitrosating acetaldehyde. That way you avoid a glycolaldehyde intermediate and produce
the (stable?) glyoxal mono-oxime which can then be converted to the bisulfite adduct.
The reaction of ethanol with nitric acid seems to essentially accomplish the same thing, but with simpler starting materials and in situ hydrolysis of
the mono-oxime. I wouldn't count out glyoxal until I'd given that method a shot.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I had an idea.
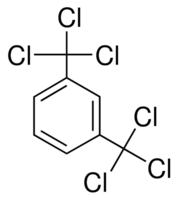
Trichloromethylbenzene is capable of chlorinating carboxylic acids. Would it be possible to chlorinate both acyl groups of oxalic acid simultaneously
with a hexachlorinated xylene?
[Edited on 25-6-2020 by JJay]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Does oxalyl chloride usually require hazmat shipping? There are resellers that could drop ship it to you.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
That would among others violate rule 7 right 
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I just had another idea, what if you make ethylene oxalate from dimethyl/diethyl oxalate and ethylene glycol. Then chlorinate it to the
tetrachloroethylene oxalate. That with either activated carbon or a tertiary amine catalyst should rearrange to two molecules of oxalyl chloride.
https://patents.google.com/patent/US2816287
https://patents.google.com/patent/US2816140A/en
I don't have a reason to make oxalyl chloride or a fume hood so I'm not about to start this  . .
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
As long as were speculating i would try doing it with Sulfur Tetrachloride with a lewis acid as a catalyst to stabilize the Sulfur Tetrachloride as
Trichlorosulfonium. Ideally the reaction would follow the first equation:
2 Cl2 + (COOH)2 + S = (COCl)2 + SO2 + 2 HCl (catalytic)
2+x Cl2 + (COOH)2 + S + 2 M = (COCl)2 + SO2 + 2 MClx•HCl (non-catalytic)
I tried making making Trichlorosulfonium Tetrachloroferrate (SCl3FeCl4) once but it failed because the iron wire did not react
fast enough (use sulfides, would probably even work with aluminium). If i had the time i would try again with Stannic Sulfide to make
Trichlorosulfonium Hexachlorostannate ((SCl3)2SnCl6) which is known to exist and requires less chlorine which is
better if the lewis acid becomes inactive by the formation of a hydrogen chloride complex (in tin's case hexachlorostannic acid). Another problem
which may be encountered is the oxidation of oxalic acid by chlorine. It could be avoided by first forming the Trichlorosulfonium and and then adding
oxalic acid though it would not be as convenient. (May be necessary anyway to regenerate lewis acid by decomposition of any Hydrogen Chloride complex)
I imagine it is a good idea to select a metal that does not form a stable oxalate either, a condition i believe tin (IV) fulfills. The last issue i
can forsee is the different temperatures needed for the chlorination too dichlorosulfur (high) and trichlorosulfonium (low?) which may prevent the
continuous process either by volatilization of the metal chloride or the instability on trichlorosulfonium, both issues are obvious how to solve.
Anyway if it works workup would be very easy, just distill off the oxalyl chloride from the metal chloride - a useful side product. I believe this
method is the most likely to work, i would love to see someone give it another try.
http://sulphur.atomistry.com/sulphur_tetrachloride.html
[Edited on 20-7-2020 by Σldritch]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It turns out to be possible to make acetylenediol diisopropyl ether from glyoxal and isopropanol:
https://www.sciencedirect.com/science/article/abs/pii/S00404...
The addition of two equivalents of chlorine gives 1,2-diisopropoxy-1,1,2,2-tetrachloroethane, which, on pyrolysis, ought to give isopropyl chloride,
2-isopropoxydichloroacetyl chloride, and oxalyl chloride, by analogy to the decomposition of the diethoxy compound described by Baganz et al in the
attached.
Thus: a phosphorous-free route entirely from published procedures.
EDIT: Ok, so Bou et al are using PCl5 to convert the glyoxal diacetals into a dichloroether. But this transformation can be achieved instead with
ZnCl2/AcCl as described by Berliner and Belecki. Also, the prep of the dichlorodimethoxyethane intermediate is apparently a unique reaction.
Plausible, but extremely laborious. There may be other ways to achieve the requisite ethylene-1,2-diether, such as an Ullmann-type vinyl
halide-alkoxide coupling.
[Edited on 12-9-2020 by clearly_not_atara]
Attachment: baganz1958.pdf (418kB)
This file has been downloaded 569 times
Attachment: bou1981.pdf (1.1MB)
This file has been downloaded 587 times
Attachment: berliner2005.pdf (103kB)
This file has been downloaded 635 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
sffap
Harmless

Posts: 7
Registered: 12-12-2018
Member Is Offline
|
|
bump
any progress on this front? I'm running out of popcorn.
|
|
|
MaeBorowski
Harmless

Posts: 12
Registered: 8-3-2021
Location: six feet under
Member Is Offline
|
|
everyone is lazy
IMHO, the most appropriate way is the decomposition of chlorination products of ethylene oxalate or ethylene carbonate.
Ethylene oxalate [poly(ethylene oxalate)?] can be easily prepared by boiling equivalent amounts of ethylene glycol and oxalic acid (you can, even
need, dihydrate) in carbon tetrachloride with a reverse Dean-Stark trap. I plan to do this synthesis sometime. 
Ethylene carbonate is synthesized in various ways, but the simplest and most accessible to the garage chemist is the interaction of ethylene glycol
with urea at low pressure and high temperature (doi:10.1039/b304182d). I find this route more advantageous, since urea is cheaper than oxalic acid,
ethylene carbonate is easy to purify by distillation even at atmospheric pressure, and yields are higher than with ethylene oxalate.
please leave comments about my English in PM
|
|
|
| Pages:
1
2
3
4
5 |