Stramonium
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
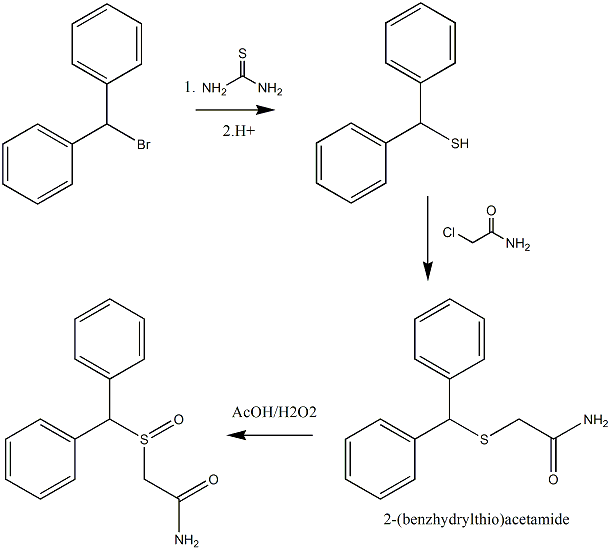
Synthesis of 2-(benzhydrylthio)acetamide
Can anyone find any issues with this synthesis-
The reaction of diphenylmethanethiol with 2-chloroacetamide to give 2-(benzhydrylthio)acetamide?
The proposed synthesis would follow a similar procedure to below [1].
"Benzhydryl-thioacetic acid
10 g (0.05 mol) of diphenylmethane-thiol and 2g (0.05 mol) of NaOH dissolved in 60 ml of demineralised water are introduced successively into a 250 ml
flask equipped with a magnetic stirrer and a reflux condenser. The reactants are left in contact for 10 minutes whilst stirring, and a solution
consisting of 7g (0.075 mol) of chloroacetic acid, 3g (0.075 mol) of NaOH pellets and 60 ml of demineralized water is then added all at once. The
aqueous solution is gently warmed to about 50°C for 15 minutes, washed with 50 ml of ether, decanted and acidified with concentrated hydrochloric
acid. after filtration, 10.2g of benzhydryl-thioacetic acid are thus obtained. Melting point 129-130°C. Yield 79%."
with the exception that an equimolar quantity of 2-chloroacetamide would substitute the chloroacetic acid to (hopefully) give
2-(benzhydrylthio)acetamide.
The 2-(benzhydrylthio)acetamide could then be oxidised with a H2O2/AcOH system to give 2-(benzhydrylsulphinyl)acetamide as detailed in [1].
Any input would be greatly appreciated!
REFERENCES
[1] US Pat 4,066,686 & 4,177,290
EDIT: changed the names of some of the compounds to remove confusion and added a reaction scheme.
[Edited on 15-5-2007 by Stramonium]
[Edited on 15-5-2007 by Stramonium]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
you can use potassium peroxymonosulfate (oxone) to selectively oxidize the sulfide and prevent oxidation to the sulfone.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by Stramonium
Can anyone find any issues with this synthesis-
The reaction of diphenylmethanethiol with 2-chloroacetamide to give 2-(benzhydrylthio)acetamide?
The proposed synthesis would follow a similar procedure to below [1].
"Benzhydryl-thioacetic acid
10 g (0.05 mol) of diphenylmethane-thiol and 2g (0.05 mol) of NaOH dissolved in 60 ml of demineralised water are introduced successively into a 250 ml
flask equipped with a magnetic stirrer and a reflux condenser. The reactants are left in contact for 10 minutes whilst stirring, and a solution
consisting of 7g (0.075 mol) of chloroacetic acid, 3g (0.075 mol) of NaOH pellets and 60 ml of demineralized water is then added all at once. The
aqueous solution is gently warmed to about 50°C for 15 minutes, washed with 50 ml of ether, decanted and acidified with concentrated hydrochloric
acid. after filtration, 10.2g of benzhydryl-thioacetic acid are thus obtained. Melting point 129-130°C. Yield 79%."
with the exception that an equimolar quantity of 2-chloroacetamide would substitute the chloroacetic acid to (hopefully) give
2-(benzhydrylthio)acetamide.
The 2-(benzhydrylthio)acetamide could then be oxidised with a H2O2/AcOH system to give 2-(benzhydrylsulphinyl)acetamide as detailed in [1].
Any input would be greatly appreciated!
REFERENCES
[1] US Pat 4,066,686 & 4,177,290
EDIT: changed the names of some of the compounds to remove confusion and added a reaction scheme.
[Edited on 15-5-2007 by Stramonium]
[Edited on 15-5-2007 by Stramonium] |
This looks like the discussion posted by Rhodium, where plenty of leads were given and references ......solo
...........source,
http://www.erowid.org/archive/rhodium/chemistry/adrafinil.mo...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Stramonium
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Thanks for both of the replies. I had seen the Rhodium synthesis but thanks for the link. A further search of a closely related forum found this
(below) which answers the questions I had. I've posted it incase anyone else has any use for it.
[Source: Posted by Antoncho Post No 429898, originally by Fomalhaut and Dionket]
benzhydryl-sulfanylacetamide.
Diphenylbromomethane (4,95g = 0.02 moles) and thiourea (1,52g=0.02moles) were refluxed in 20mls water for 30mins. As the synth from Rh's says, a clear
solution must have been formed in 5 mins, but in the end we still had a lot of oil at the bottom (the reasion to blame was old, semidecomposed
diphenylbromomethane - when we opened the can, it emitted HBr). We were too lazy to separate the oil , so 2.5g (0.04moles) KOH in 15mls water was
added straight and the reflux continued for 30 more mins. A disgusting stench filled the lab.
Thus obtained solution of potassium salt of diphenylmercaptane was cooled to 50-60 C and 1.9g (0.02moles) of chloroacetamide was added thereto. The
mixtr was left to its own devices for 2hours - the precipitated oil crystallized. The xtals were filtered, washed thrice w/water, thrice w/ether
(removing all benzhydrol). After drying there was obtained 1.9g (37%) of finely divided crystals with mp of 111 C.
With fresh diphenylbromomethane this will give not less than 80% - otherwise I'll bee a reddish (this is an idiom which I am again unable to
translatesmile).
Modafinil
Into the solution of 3.6g benzhydrylsulfanylacetamide (0.014moles) in 15mls of GAA there was added 3mls (~0.03moles) 30% hydrogene peroxide. The
mixture was left at RT (15 Ñ in our case, better not to heat above) for 20 hrs. Then into the solution there was added 30mls aqua, scratching the
walls with a glass rod. After 1 hr the precipitate was filtered, washed w/water twice, then w/ether and dried. Yield - 2,3g (61%), mp - 158-159 C.
After some time the mother liquor yielded some more product but we were too lazy to work it up.
|
|
|
heksogen pl
Harmless

Posts: 5
Registered: 17-1-2010
Member Is Offline
Mood: No Mood
|
|
Sorry for digging out a dinosaur,
I'm currently searching for a synthesis of diphenylmethyl bromide from diphenylmethanol which is easily obtainable from aldrich.
This seems a relatively easy SN1 reaction, but the workup would be different than in synthesis of other aliphatic bromides - the product is a solid
MP= 45°, BP= 184° @ 20 mm Hg, while the corresponding alcohol has MP = 65-67*C, BP= 298*C @ 780mm Hg making it rather hard to isolate via vacuum
distillation.
I wonder if I could extract the product with toluene - but again cannot find any info about solubility of diphenylmethanol (alas, seems hydrophobic).
Maybe adding toluene to the reaction mixture (48% HBr, H2SO4, diphenylmethanol) and using very rapid stirring would simplify the procedure and move
the equilibrium towards product.
I'll try digging beilstein, but maybe someone has some valuable info ?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You can use acetic acid as solvent and about 3-fold excess of 48% or 62% HBr (whichever you have). Then just heat for a dozen of minutes on a steam
bath, check with TLC, if finished dilute with water and crystallize the dropped out oil by using whatever solvent is recommended (for benzhydryl
bromide I would guess methanol is used). Never really used this on a benzhydryl alcohol, but it works satisfactorily on benzyl alcohols which are much
less prone for SN1 substitutions.
PS: I don't know what you need benzhydryl bromide for, but you should consider that many nucleophiles can be easily alkylated with benzhydyl alcohol
just as easily (under acidic conditions in SN1 substitutions).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
heksogen pl
Harmless

Posts: 5
Registered: 17-1-2010
Member Is Offline
Mood: No Mood
|
|
I will use it for synthesis of modafinil or its analogs (too much work to do to waste time for sleeping  ). I haven't seen any synthesis of diphenylmethanethiol using benzhydryl alcohol, though there is a mention about
direct synthesis of thiols from corresponding alcohols, thiourea and HBr (5.17.1, page 754, Vogel 5th edition) ). I haven't seen any synthesis of diphenylmethanethiol using benzhydryl alcohol, though there is a mention about
direct synthesis of thiols from corresponding alcohols, thiourea and HBr (5.17.1, page 754, Vogel 5th edition)
Thanks for help nicodem.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
You can make the starting halide as well through the friedel crafts alkylation of benzene with CHX3. Do the reaction in an excess of chloroform (or
bromoform if you must). The amount of AlCl3 doesn't matter, I have had luck with between .1 and 1 eq. You know the reaction is done when the
evolution of HCl ceases. I did this reaction many times on p-xylene with chloroform. Once initiated the reaction is pretty quick even at ice bath
temperatures (appreciably done in an hour). I expect the reaciton will proceed pretty quickly with benzene. Just a note, it fails with
p-methoxyanisole, at least at room temp, with even a >>2x excess of AlCL3.
With para-xylene, upon hydrolysis, the mix becomes blue fluorescent, I never isolated this impurity (trace by tlc), it might be the
9,10-dichloroanthracene, or the 9-chloroanthracene. Based on this, I would not try to heat the reaction to try to squeeze out better yields from this
reaction (the yields are already pretty good anyways).
[Edited on 1-17-2010 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
heksogen pl
Harmless

Posts: 5
Registered: 17-1-2010
Member Is Offline
Mood: No Mood
|
|
@ smuv :
Unfortunately, despite toxicity of benzene, the friedel-crafts reaction can go further forming disubstituded benzene (Steric hindrance won't preserve
it from going into para position). To worsen the case, the first substituent will activate the ring.
Benzene and dibromophenylmethane (benzal bromide) should be used to form the mentioned bromide.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
You have no idea what you are talking about, I am speaking from real world experience.
The alkylation with chloroform is a special case, because the benzal chloride formed is very deactivated and will not undergo further alkylation.
Even the possible presence of the anthracene derivatives is surprising, in light of how ring deactivated the phenylhalomethanes are.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
heksogen pl
Harmless

Posts: 5
Registered: 17-1-2010
Member Is Offline
Mood: No Mood
|
|
Yes I do know the reaction, it is the intermediate of Reimer-Tiemann reaction. It was my mistake, sorry. But again - I'm supprised that the
diphenylmethyl halide does not undergo another electrophilic attack.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by heksogen pl  | | I haven't seen any synthesis of diphenylmethanethiol using benzhydryl alcohol, though there is a mention about direct synthesis of thiols from
corresponding alcohols, thiourea and HBr (5.17.1, page 754, Vogel 5th edition) |
Thiourea is a powerful nucleophile also in acidic media and can be thus used in SN1 substitutions. See WO2008031227 or Bulletin of the Chemical
Society of Japan, 77, 2011-2020 for the synthesis of benzhydrylthiol from benzhydryl alcohol.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
It think it doesn't make any sense to use oxone instead of hydrogen peroxide to prevent over-oxidation to the sulfone. (James R. McCarthy, Donald
P. Matthews, and John P. Paolini (1998), "Reaction of Sulfoxides with Diethylaminosulfur Trifluoride", Org. Synth.; Coll. Vol. 9: 446)
It might be useful to add hydrogen peroxide in the presence of a alcohol & mineral acid and at low temperature. (US patent 7057068)
I think you can reduce the sulfone to the corresponding sulfoxide (modafinil) by using a reducing agent like NaBH4 Not sure or side reactions would take place like a too strong reduction, which might give rise to the previously obtained sulfanyl
(sulfide) structure?
Good to be wrong
|
|
|