| Pages:
1
..
16
17
18
19
20
..
44 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Excellent! That is a good conservative way to do it. It does rather leave Vogel standing in the corner wearing a pointy hat, doesn't it?
Do you have enough SOCl2 left to try to react it directly with dry NaOAc to see if Ac2O can be had that way as well?
Sic gorgeamus a los subjectatus nunc.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Congratulations! excellent work!
Now this is the way of concluding on a reaction working or not. Sauron gave a very detailed theoretic description, very interesting, but your
experimental work gave irrecovable results.
I only wish more people acted like you, Engager, trying out experiemntal work even if details are lacking in the litterature. It seems alot of people
are too reticient to "waste" their reagents unless there is a very detailed, established procedure.
Thanks for sharing. I'm sure many will appreciate
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There was no doubt that SOCl2 and AcOH would yield AcCl; Vogel and others merely quibbled about the difficulties of isolating the AcCl in pure form -
especially in the days when SOCl2 was usually contaminated with various P and S compounds.
And there was no reason to doubt that AcCl would react with anhydrous NaOAc. Various people have done a lot of pissing and moaning about the chore of
fusing the trihydrated acetate twice and losses particularly in the second fusion.
I have always argued for buying the best grade of anhydrous sodium acetate like super dry Merck and using freshly opened bottles all at once. But then
people piss and moan about the higher price.
There's a lesson in human nature for you.
I am working on a non-SO3, non-oleum and non-sulfur chlorides method for producing practical quantities of SOCl2 from SO2 and hexachloro-m-xylene
using FeCl3 (anhydrous) as catalyst. In this country SOCl2 is banned so making it is only option and oleum is too bloody expensive. HCMX is not so
hard to prepare. See the thread on this project. (SO2Cl2 -> SOCl2 Some Musings).
There is also reason to expect that SO2 will react with benzotrichloride and catalyst albeit more slowly. Benzotrichloride is commercially available.
HCMX is too but horribly expensive.
Len1 tried this but used wrong catalyst, negative results.
Kyrides teaches that SOCl2 can be made from phthaloyl chloride and SO2 at 200 C over ZNCl2 in 66% yield.
So anyway we are closing in on more convenient preps of SOCl2 that can be done in glassware and that will be good news.
[Edited on 26-8-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
@ Sauron: what you're saying seems in good accord with the patent procedure. I should have expounded on the process with a few more details so below
is the translation of the patent process. In the second example they use AcCl, in the other trial part with SOCl2 they just substituted the AcCl below
with 119 parts technical grade SOCl2, and using 180 parts AcOH also instead of the amounts below, to get Ac2O:
In a fractionating column and reflux condenser which is attached to it, which coolant is held at -15 to -20º, 200 parts glacial acetic acid are kept
at boiling, so that the column is filled with acetic acid vapors. Then 131 parts of acetyl chloride are gradually added through a pipe which leads
into the boiling acetic acid, and further heated until the hydrogen chloride evolution ceases showing the end of the reaction. Then the liquid left in
the boiling vessel is subjected to a careful fractionated distillation yielding pure acetic anhydride. Yield: 90% of the theory.
| Quote: | Originally posted by Klute
Congratulations! excellent work! |
Yeah, second that. I also like seeing reactions get put to the test.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
AcOH and SOCl2 will give AcCl; AcCl will react with AcOH to form Ac2O. Nolo contendere.
The part I was barfing on, for lack of lit. support, was NaOAc + SOCl2 -> Ac2O. However, I am not prepared to say this does not work. I just
doubt that it does. I don't think SOCl2 works same as oxalyl chloride, mechanistically, that is, through a double anhydride stage. So no reason to
assume parallel reactions.
I'd like to see someone try it though. I can't - no SOCl2 and in Thailand it's verboten. So it would have to await my SOCl2 synthesis.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by Sauron The part I was barfing on, for lack of lit. support, was NaOAc + SOCl2 -> Ac2O. However, I am not prepared to
say this does not work. I just doubt that it does. I don't think SOCl2 works same as oxalyl chloride, mechanistically, that is, through a double
anhydride stage. So no reason to assume parallel reactions |
Can't say about SOCl2, but Beilstein cites a reference for the technical preparation of acetic anhydride using anhydrous NaOAc and sulfuryl chloride:
G. Cohn in F. Ullmann, Enzyklopädie der technischen Chemie, 2. Aufl. Bd. IV [Berlin-Wien 1929], pg. 690, and maybe also something in: Gassner
G.m.b.H., Häusler, McLang, Chem. Trade J. 76 [1925], 787. Beilstein also mentions pyrogenic dehydration of AcOH from DE 486953 (just the jist as
given by Beilstein): the mixture of acetic anhydride vapor and water vapor is bound by addition of compounds, which builds azeotropic mixture with the
water, then following condensation of anhydrous Ac2O.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Yes but as you say. That's SO2Cl2. Despite the similarity in formula, these two compounds have vastly different chemistries. And they are not so easy
to interconvert. SO2Cl2 mostly reacts as a stabilized form or source of SO2 and Cl2. Hence its employement with radical initiators as a free radical
chlorinating agent. Sulfuryl chloride in ordinary temperatures is not a dehydrating agent. It can be mixed with cold water. (See entry in Merck.)
Formally SO2Cl2 is the acid chloride of H2SO3 and SO2 is the anhydride of that acid (sulfurous acid.) This is a convenient way of looking at it, but
sidesteps the inconvenient detail that H2SO4 only exists in rather dilute aqueous solutions. So one could prepare SO2Cl2 rather expensively from SO2
and oxalyl chloride. Just mix and let the CO and CO2 evolve, reflux everything else. But why bother when just mixing SO2 and Cl2 over activated
carbon or camphor is just as effective and a lot cheaper?
Anyway I think it's a mistake to draw any inferences from those citations re SO2Cl2, especially since they long ago vanished as industrial methods.
I would not assume that they were understood mechanistically at the time.
Simple enough to try NaOAc and SOCl2 if one has them at hand.
We do know NaOAc and oxalyl chloride works, because Roger Adams recommended it in 1918 in JACS.
[Edited on 27-8-2008 by Sauron]
Attachment: RAdams.pdf (1.1MB)
This file has been downloaded 927 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Sauron, you say sulfuryl chloride, SO2Cl2 , is the acid chloride of sulfurous acid. I'm almost sure it's not, it's the acid chloride of sulfuric acid
(S is in 6+ oxidation state, each OH-group in H2SO4 replaced by Cl). Hydrolysis of sulfuryl chloride will result in sulfuric acid and HCl. I'm not
sure, but I think action of SO2Cl2 on methanol yields dimethylsulfate. It will at least form methyl chlorosulphate/methylsulfuric acid
SOCl2 is the acid chloride of sulfurous acid.
Can't acetic anhydride be prepared by means of action of CO upon methyl acetate, or does this reaction require veryy high temperature/pressure, or
expensive catalysts?
[Edited on 22-9-2008 by Jor]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
H2SO3 only exists in dilute solution and cannot be isolatred.
Chlorinating SO2 produces sulfuryl chloride if elemental chlorine is reacted with SO2 over AC or camphor.
So thanks but the correction is rather useless.
Chlorinating SO2 with phthalyl chloride does produce thionyl chloride.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by S.C. Wack
| Quote: | Originally posted by hector2000
this method is not true
no anhydrid will be make
|
Ber. 9, 444 (1876): "Nach Versuchen der HH.H. Gal und A. Etard kann Essigsäure unter günstigen Umständen durch Phosphorsäureanhydrid in
Essigsäureanhydrid umgewandelt werden. Man trägt ziemlich rasch 30 Grm. Phosphorsäureanhydrid in 60 Grm. Essigsäure ein, indem man durch
beständiges Schütteln dafür Sorge trägt, dass die beiden Substanzen sich möglichst schnell vermischen. Das Gemenge wird bald braun und erhitzt
sich etwas, in diesem Momente destillirt man rasch ab und isolirt das gebildete Essigsäureanhydrid durch die fractionirte Destillation. Die Verfasser
haben auf diese Weise ungefähr 3 Grm. der letzteren Verbindung erhalten. Benzoësaure auf ähnliche Weise mit Phosphorsäureanhydrid behandelt
liefert eine geringe Menge Benzoësaureanhydrid."
OK so the yield is a little low if true. This is not the same as "no". IIRC Étard killed himself, but not over cries of bullshit on this.
|
P2O5 could be used decently indirectly, to the preparation of Ac2O, by using either of the following: 1. Ber., 10, 1790 mentions passing dry HCl for
2 hrs into a mixture of P2O5 and GAA held at 0ºC during that time. The P2O5 absorbs the H2O which normally destroys the AcCl. The liquid is then
distilled, AcCl is said to form in significant amounts. 2. Using P2O5 which can be heated with NaCl to form POCl3 (mentioned briefly here), which can be used to get the AcCl: 2 CH3COONa + POCl3 -> 2 CH3COCl + NaPO3 + NaCl. Then use the AcCl to get the Ac2O.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
"Mentioned briefly here"? The reaction of P2O5 and NaCl as described by Tarbutton in JACS, has been flly described here and the original paper posted
by me. You make it sound like a trivial operation. In fact that reaction does not initiate below 400 C, requires a high pressure apparatus (autoclave)
to handle the autogenous pressure and takes about 20 hours on even a modest scale. There are a lot easier ways to make acetyl chloride than to prepare
POCl3 to make it - which is not a very good method anyway.
Few of us happen to have a large pressure reactor sitting around. Most people prefer to work in glass. There are at least half a dozen good methods
for preparing AcCl that run fine in pyrex, and have been described on this forum.
As to the proposition of GAA and P2O5 being treated with anhydrous HCl, that is fine if you happen to have a large cylinder of dry HCl handy. I have
been pricing control valves and regulators for exactly such a cyclinder and find that they cost about 6 times the price of brass regulators for
noncorrosive gases. That is, about $600 US for the cheapest single stage stainless steel regulator I have found. So do you really think this is a
practical approach after all? That is, assuming that the method works at all?
You can prepare acetyl chloride from:
GAA and TCT (cyanuric chloride) in acetone in presence of TEA
GAA and benzoyl chloride (1.5 to 2 excess of reagent) per H.C.Brown in JACS
GAA and phthaloyl chloride per L.F.Kyrides in JACS
GAA or anhydrous sodium acetate and oxalyl chloride per Roger Adams in JACS
or from GAA and Ph3P in CCl4
Or you can go straight to Ac2) from anhydrous sodium acetate, bromine and sulfur; or from oxalyl chloride and anhydrous sodium acetate in different
proportions than for preparing AcCl.
Most assuredly if I were to go to the trouble of making POCl3 I would not waste it on preparation of acetyl chloride for which so many other less
precious reagents and simpler methods are at hand.
The same is true of thionyl chloride.
There arer several more mild neutral preps of acetyl chloride and other acyl chlorides, likewise described here previously.
[Edited on 25-9-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Well, there is the possibility that you can produce acetic anhydride, simply by mixing glacial acetic acid, with isopropenyl acetate, and
distilling off acetone.
I haven't heard that isopropenyl acetate is restricted.
Am I missing something?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
What is the basis for your assertion that Ac2O can be had by distilling GAA with isopropyl acetate?
Put some literature forward, please. Because I see nothing at all to this idea. I think it is nonsense.
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Isopropenyl Acetate. AKA Methyl Vinyl Acetate. The enol ester of acetone. You know, the one that is created by reacting acetone with ketene.
U.S. $54.20 a liter via Aldrich. Product Number 117781
Interesting stuff.
It is used as an acetylating agent. It can acetylate cellulose, and In fact, it may be used to manufacture anhydrides.
So, you're kidding me right? You always did have such a wonderful sense of humor.
It really never occurred to you, that you might be able to produce acetic anhydride by mixing Isopropenyl Acetate with Glacial Acetic Acid? And
then, drive the reaction to completion by removing acetone as it forms?
Think about it.
http://www.britannica.com/EBchecked/topic/25412/anhydride
http://www.informaworld.com/smpp/content~db=all~content=a757...
http://www.freshpatents.com/Method-for-the-production-of-ace...
[Edited on 12-10-2008 by zed]
[Edited on 12-10-2008 by zed]
[Edited on 12-10-2008 by zed]
[Edited on 13-10-2008 by zed]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Isopropenyl acetate does indeed acetylate acetic acid to form acetic anhydride, but as far as I remember you need a catalytic amount of TsOH or other
acids to reach equilibrium fast enough. Just refluxing acetic acid with isopropenyl acetate without acid catalyst and removing acetone trough
fractionation might take too long. Anyway, Sauron and Hector already made a looong discussion on the subject. There were patents posted about this
reaction using the cheaper vinyl acetate instead. Isopropenyl acetate is twice the price of acetic anhydride.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Price is not the problem. A lot of the guys would like to have a little acetic anhydride.
Making it from Isopropenyl Acetate might be cheap enough, and probably relatively safe.
Here in the U.S., I think I could obtain A.A. fairly easily. In other parts of the world, closer to the poppy fields, it might be very tough to
acquire.
As for fractionating, I do not know if it would be required. Acetone has a much lower boiling point than Isopropenyl Acetate, Acetic Anhydride, and
even Glacial Acetic Acid.
And, since we are talking about an equilibrium.....It is conceivable that the acetone might be removed from the reaction mixture below normal reflux
temperature, via vacuum distillation. Thereby reducing the formation of byproducts.
Having read the Hector thread, and the 18 pages of this thread, I did not recall any mention of Isopropenyl Acetate. So, I figured maybe it had been
overlooked.
[Edited on 13-10-2008 by zed]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Isopropenyl acetate = methyl vinyl acetate, all right. If you go back far enough in the thread you will see that I posted several papers dealing with
vinyl acetate in similar reactions, also involving catalysts. Somewhere around here I have a large bottle of stabilized vinyl acetate monomer, which I
purchased for the purpose of trying this out. However I have not yet done so as for various reasons my hood is not yet installed.
I assume methyl vinyl acetate also is a monomer requiring stabilization and therefore needs distilling prior to use in this reaction? '
I await someone actually performing this reaction and reporting success and preperative details.
Till then it seems to me there are quite a few better reactions out there than this one. Put not your faith in patents, sayeth Sauron. They'll bugger
you every time.
[Edited on 13-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Sauron
"Mentioned briefly here"? The reaction of P2O5 and NaCl as described by Tarbutton in JACS, has been flly described here and the original paper posted
by me. You make it sound like a trivial operation. In fact that reaction does not initiate below 400 C, requires a high pressure apparatus (autoclave)
to handle the autogenous pressure and takes about 20 hours on even a modest scale. There are a lot easier ways to make acetyl chloride than to prepare
POCl3 to make it - which is not a very good method anyway. |
I cited it as a reference where it was only briefly mentioned there. Always good to know further details.
| Quote: | | As to the proposition of GAA and P2O5 being treated with anhydrous HCl, that is fine if you happen to have a large cylinder of dry HCl handy. I have
been pricing control valves and regulators for exactly such a cyclinder and find that they cost about 6 times the price of brass regulators for
noncorrosive gases. That is, about $600 US for the cheapest single stage stainless steel regulator I have found. |
Anhydrous HCl can be simply generated from:
a. conc. HCl and H2SO4. I had a glass appartus scheme for this, but I'd have to search for it.
b. mixing NaCl and H2SO4 which foams up a bit (overflow potential) releasing HCl, this mixture can later be heated to increase rate of reaction: NaCl
+ H2SO4 = NaHSO4 + HCl.
And then likley by melting NaHSO4 and NaCl (both dry). I've used this mixture to generate HCl, but not from anhydrous salts.
| Quote: | | So do you really think this is a practical approach after all? That is, assuming that the method works at all? |
I don't think Berichte publishes shady papers. The paper cites Friedel in a Compt. rend. paper who carried the reaction out at 80º, the Berichte
paper brought the reaction down to a lower temperature.
| Quote: | | Originally posted by Jor Can't acetic anhydride be prepared by means of action of CO upon methyl acetate, or does this reaction require veryy
high temperature/pressure, or expensive catalysts? |
Carbonylation of methyl acetate is a known method of Ac2O production. US4544511 describes synthesis using MeOAc and Me2O but they do use catalysts and
autoclaves.
| Quote: | Originally posted by zed
Isopropenyl Acetate. AKA Methyl Vinyl Acetate. The enol ester of acetone. You know, the one that is created by reacting acetone with ketene.
|
If you intend on using ketene to get isopropenyl acetate, then why not just use ketene directly with GAA to get acetic anhydride?
[Edited on 13-10-2008 by Formatik]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
A pile of preposterous, impractical and unsafe blather.
Obviously you have a perculiar idea of what "anhydrous" means. If you generate HCl by wet methods you will need to scrub the gas to remove acid mist
and some residual moisture. TANSTAAFL, after all. That what I mean by a handy cylinder.
We wasted many pages already arguing about ketene generation at home, it's an asinine idea, so don't do it. Furthermore since GAA can be used as a
feedstock for ketent I suggest that the cited patent process will also produce some ketene, so same injunction applies. Of course if you are just a
paper chemist and don't actually DO anything, then feel free to cite all the unworkable crap you care to and propose all the $100 million dollar
industrial processes you want to. But not one drop of Ac2O will come of it.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here is the schematic for HCl being referred to. It's described in Lehrbuch der anorganischen Chemie by Hugo Erdmann pgs. 299-300. Heating fuming HCl
acid, and then drying with pumice drenched with conc. H2SO4. A more complex set-up is described up until page 301 using a Kipp’s apparatus. Simpler
would be to use the other suggestions.
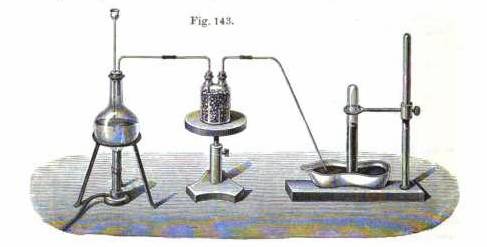
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
YAWN. Wake up and smell the coffee, this is the 21st century not the 19th. Perhaps you want to go back to using alchemical symbols as well?
What is HCl and P2O5 supposed to fetch you? POCl3? In what time frame? Do you think a mixture of POCl3 and P2O5 just sits there and happily coexists?
Sorry. They react with each other. As far as GAA is concerned you might get a little acetyl chloride, but then what? It'll be a bitch to seperate it
from all that POCl3/P2O5 mess.
In short this is not a useful and practicable method, I don't see any Ac2O coming out of it, there are better ways to prepare POCl3 and there are lots
of better ways to prepare Ac2O. If you'd spend a little time reading the pertinent threads you'd know that.
"Fuming hydrochloric acid"? All hydrochloric acid (conc) fumes. The term is meaningless. Fuming sulfuric acid is any sort of oleum of which common
concentrations of dissolved SO3 run 20 to 65 per cents. Fuming nitric acid is any sort of nitric acid above d.1.42 up to a bit over 1.50. Fuming
hydrochloric? It's redundant.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by Sauron What is HCl and P2O5 supposed to fetch you? POCl3? In what time frame? Do you think a mixture of POCl3 and P2O5
just sits there and happily coexists? Sorry. They react with each other. As far as GAA is concerned you might get a little acetyl chloride, but then
what? It'll be a bitch to seperate it from all that POCl3/P2O5 mess. |
Beilstein was saying something about dehydration, the Berichte paper describes the excess P2O5 acts on CO.OHHCl groups and converts it to the
chloride, but they also hold it possible to work dehydrating.
| Quote: | | In short this is not a useful and practicable method, I don't see any Ac2O coming out of it, there are better ways to prepare POCl3 and there are lots
of better ways to prepare Ac2O. If you'd spend a little time reading the pertinent threads you'd know that. |
Very well.
| Quote: | | "Fuming hydrochloric acid"? All hydrochloric acid (conc) fumes. The term is meaningless. Fuming sulfuric acid is any sort of oleum of which common
concentrations of dissolved SO3 run 20 to 65 per cents. Fuming nitric acid is any sort of nitric acid above d.1.42 up to a bit over 1.50. Fuming
hydrochloric? It's redundant. |
Well then we know not below concentrated is best suited.
[Edited on 13-10-2008 by Formatik]
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Formatic,
"If you intend on using ketene to get isopropenyl acetate, then why not just use ketene directly with GAA to get acetic anhydride?"
I never suggested using ketene. My suggestion was to buy some Isopropenyl Acetate, and then use it to prepare a modest amount of acetic anhydride.
Isopropenyl Acetate is fairly safe to handle.
As for anhydrous HCl, It is readily generated by the action of Concentrated H2SO4....on rock salt. Cheap and easy. The expense is inconsequential.
I would never consider buying HCl by the cylinder. Very expensive to work with, and here in the States, it might be considered a suspicious
acquisition.
Sauron,
You have some vinyl acetate? Hmmm. Might it be oxidized to acetic anhydride via the Wacker? Or, has this option already been discussed?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ketene in a home lab = DEATH. If you bother to study the cumulative knowledge base on Ac2O you will see that it is not necessary to kill yourself to
make Ac2O.
Ketene is good in an industrial setting, not in a home lab. FORGET ketene or proceed at your own peril.
You have literature on a Wacker oxidation of VA -> Ac2O?
If not I suggest focusing on the dozsen or so good practicable methods that are suitable for a home lab.
NOT industrial processes. Ac2O is highly flammable unlike GAA and at 800 C is a serious problem from so many standpoints: corrosiveness,
flammability, toxicity. You wanbt to deal with superheated Ac2O vapor in your home? Not me.
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ah, a misunderstanding. I'm not talking about the Wacker process for producing Acetic Anhydride Via Ketene.
I'm suggesting that Acetic Anhydride might be produced by the Wacker oxidation of terminal alkenes. Like Vinyl Acetate. This would be analogous to
the Wacker oxidation that is performed on terminal alkenes, to produce Methyl Ketones. A reaction that proceeds under mild conditions.
Since Vinyl Acetate is often made by the reduction/dehydration of Acetic Anhydride, it is reasonable to conjecture that Vinyl Acetate might be
reoxidized, back into Acetic Anhydride.
No home lab here. I either build a real lab, or facsimile thereof, or I visit the Uni.
It is important for a gentleman, to avoid the appearance of possible impropriety.
Moreover, as an organic chemist, it is important to avoid working without a modern fume hood.
|
|
|
| Pages:
1
..
16
17
18
19
20
..
44 |