| Pages:
1
2
3
4
5
6
..
13 |
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Yes I know well back to sleuthing. No I did not intend to suggest using sodium fluoride in the doping of baked coatings. I made a reference to the
addition of 1 or 2 grams of sodium fluoride to the sodium chloride to be electrolyzed with lead dioxide anodes.
No, I would add the 2% by weight by adding ammonium bifluoride to one of the nitrate solutions. I have 8 ounce of this fluoride I plan to use for
etching, fluxing, or glass etching.
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Well, my antimony, tin and bismuth finally arrived today. They were rattling around in the bottom of a cardboard box, with balls of paper on top, they
weren't labelled so I hope I can identify them correctly ... 
They took sooo looong, I think they went by mule train via Outer Mongolia. Should be enough here for at least 5x10^4 anodes, should I choose to make
them ... 
There is about 1 Kg of each, the Bi at the bottom of the image is about 10 cm across, it sure is heavy!
[Edited on 22-1-2008 by Xenoid]

|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
You should be able to differentiate them by their densities, Bi being much denser than the others although still only about 25% denser than Fe. Also,
Bi has an orange tinge, and Sn is softer than Sb and Bi.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@ Xenoid: Mind your toes.
Don't know what thread you mentioned the problem with Methylene blue and warm test solution but I will stick this here.
Took two test tubes and put a solution of Perchlorate that was giving a fairly positive test for Perchlorate. Heated one to about 40C. It will not
give an indication for Perchlorate when Methylene blue dropped in.
Put in some more perchlorate to solution. Filled two new test tubes. This time the test for Perchlorate was very very positive in the cold tube. The
one heated to approx. 40C did not give a good positive indication.
Lesson: (as pointed out by Xenoid) Make sure your Perchlorate solution is cold when testing for Perchlorate with Methylene blue.
Dann2
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
My Fixture
Well, I finally got my act together and put a decent tube furnace fixture in action. As you can see I found four fire bricks that PERFECTLY fit
around my aluminum tube. It is almost that I planned the bricks to work except I purchased them 9 years ago!  I played with the four baffles in the gun and
if I blocked 2 I could get 350°C and if I block 3 I get 427°C at the end of the tube! I played with the four baffles in the gun and
if I blocked 2 I could get 350°C and if I block 3 I get 427°C at the end of the tube!  And that is at an outdoor reading of -9°C And that is at an outdoor reading of -9°C My toes are frozen!! My toes are frozen!! I am doing my first cobalt coating as I write this on the lower temp. I will use the 427°C for the 1 hour bake. Heres the pick,
Enjoy! I am doing my first cobalt coating as I write this on the lower temp. I will use the 427°C for the 1 hour bake. Heres the pick,
Enjoy!
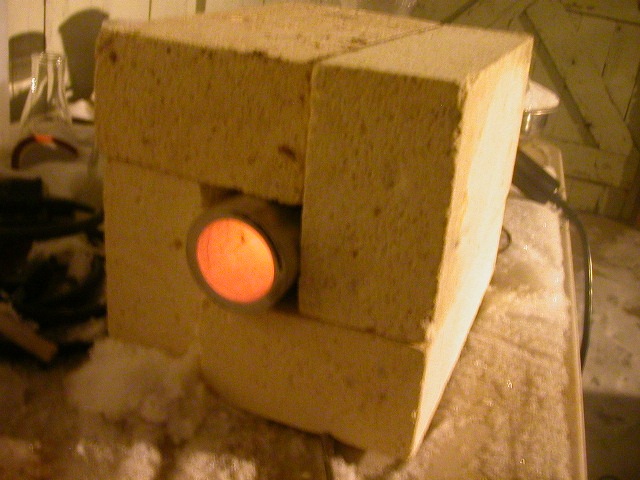
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ chloric1
That looks pretty damn hot in there ... 
How are you mounting the rod(s) with a horizontal baking arrangement?
Christ! Is that snow/ice on the bench!
[Edited on 24-1-2008 by Xenoid]
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Yes there is snow and ice everywhere! A sunny day at 5°C would be very balmy to me right now.
The hell glow is a deceiver. Illumination from the heating element makes it look 800°C but the tube is 6061 aluminum . Now I am running a second 10 minute coat. . Now I am running a second 10 minute coat.
I might run out of time here I think I'll try a third coat with nickel nitrate and
then on to the 1 hour bake cycle. The other anode I plan on making I will do the 7 coats if I can find the time. I think I'll try a third coat with nickel nitrate and
then on to the 1 hour bake cycle. The other anode I plan on making I will do the 7 coats if I can find the time.
Yes it is horizontal. Basically, I support the rod on a ring stand using a clamp that allows you to mount shafted devices perpendicular to the
support shaft. Pictures will explain far better.
Let me put more pictures on here tomorrow.
[Edited on 1/24/2008 by chloric1]
Fellow molecular manipulator
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I scoured my Ti strip clean today, gave it a nice warm bath in HCl, and 3 coats of a more dilute Co(NO3)2 than I had been using - less excess to rub
off after each coat. I made up a new solution for ATO, when I've done this, I let the HCl dissolve ALL the tin, leaving the SbO at the bottom, then
add a splash of H2O2, the whole solution foams up like beer, and everything is dissolved, happy, and presumably the SnCl2 is now SnCl4 - assuming
there is enough HCl in solution to permit this. Given the level of foaming, I think there is at least a good percentage of SnCl4.
Once I've completed baking this crap on, I will be attempting the PbO2 plating again. I may do another strip with Co spinel and ATO while the solution
is still fresh.
I've been reusing my Ti etching solution, I happen to have 6 250ml graduated cylinders so I just left ~180ml in one of them, cover it with plastic
wrap and a rubber band. I just set it in my sink, resting at an angle, and run hot water over it to heat it up, uncover a bit and slide in the Ti,
recover.
Chloric: 5C is like, tropical! It's been around -35C here for the last few weeks, sometimes colder with the wind chill.
Xenoid: after 2 coats of Co spinel the weight was 13.9g and the thickness 1.01mm - see! I remembered this time. I'll weigh after the DTO coats and see
if there is any difference.
[Edited on 26-1-2008 by tentacles]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Do you intend to test the DTO coatings to see what they are capable of withstanding?
I have feeling that they will fail (Ti will passivate) very quickly in a Chlorate or Perchlorate cell.
You would need to test a DTO only on Ti to see as the Co Oxide will stand up for a few hours in a Perchlorate cell and some days in a Chlorate cell.
I would be interested to see if you are getting 'good' DTO coatings from the method you are using. By 'good' I mean a coat that will last for say 10
hours (or even days) in a Chlorate or Perchlorate cell.
Perhaps there is no need for such a good coat when all we are asking it to do is be an undercoat?
Dann2
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I ended up making 3 anodes today, the first one (that I spoke of above) is getting it's plating of PbO2 now. The other two I sanded, etched, and
hydrided. Then applied 4 coats of Co spinel, and one coat of DTO over the Co spinel. I'm not sure if I am going to put another on as the DTO is
getting kind of dirty. The anode getting plated now has 5 coats of DTO on it, and I did notice that very little rubbed off after each baking.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
.... then add a splash of H2O2, the whole solution foams up like beer, and everything is dissolved, happy, and presumably the SnCl2 is now SnCl4 -
assuming there is enough HCl in solution to permit this. Given the level of foaming, I think there is at least a good percentage of SnCl4.
|
Do we know for sure that this actually works? I have scoured the internet and various chemistry books, I have found several procedures for SnCl4, but
I have not been able to find this method described.
I recall seeing the original post, around the middle of last year, I wasn't interested in anodes at that stage, so I didn't follow it very closely!
Can someone point me to the original post. Otherwise, I will have to UTFSE.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
From *everything* which I have read , the indications
are that except as an intermediate along the way to something else , chlorides are *not* the best choice
of a direct pre-bake precursor for DTO . In those schemes where the chloride was used there was more elaborate methods employed which put the
*actual* intermediate beyond being a scenario where it was simply " dip substrate in chloride solution precursor and bake " .
Spray pyrolysis yes , dip and bake ......no way .
That is an oversimplification which does not square with
the chemistry required .
Where the chlorides have been used in any dip and bake schemes , the "digestion" of that chlorides precursor with
alcohol or simply it's keeping for some time , produces a
conversion from any "clear solution" of chlorides into a
dispersion colloid , a hydrosol , with loss of HCl and oxidation
to a dispersed form of probably metastannic acid . And the pH of that dispersion has great bearing on how well or poorly it adheres when it is baked
, what quality of coating
it produces .
[Edited on 26-1-2008 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Xenoid, Xenoid, Xenoid. I am absolutely, completely and totally disgusted with you not reading (and forever remembering) the original post on
converting SnCl2 to SnCl4.
It was a mere 2 months ago (though it was far longer meself).
http://www.sciencemadness.org/talk/viewthread.php?tid=2465&a...
Some here too:
http://www.sciencemadness.org/talk/viewthread.php?tid=9569
All attemps at making ATO coatings using SnCl2 or 'SnCl4' (from SnCl2 + H2O2) [+ Sb liquid] failed for me. I was testing coatings in a Chlorate cell.
Got ATO coating to work OK (as an anode, did not get around to plating PbO2 yet) using SnCl4:5H20 (out of a jar) + Shake and Bake As per the Diamond Shamrock pats. As per the Diamond Shamrock pats.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
| Quote: | Originally posted by Rosco Bodine
........... for DTO . In those schemes where the chloride was used there was more elaborate methods employed which put the *actual* intermediate
beyond being a scenario where it was simply " dip substrate in chloride solution precursor and bake " .
Spray pyrolysis yes , dip and bake ......no way .
That is an oversimplification which does not square with
the chemistry required .
|
What do you mean by this?
It has been stated to work OK in lots of the patents.
ALL my failures were using SnCl2 (SnCl2 is used in none of the patents, it is available on ebay though).
I got ATO to work OK (not as a productive anode as such) for some weeks in both Chlorate and Perchlorate cells. Seems like a 'not too bad' undercoat
between LD and Ti to me.
| Quote: |
Where the chlorides have been used in any dip and bake schemes , the "digestion" of that chlorides precursor with
alcohol or simply it's keeping for some time , produces a
conversion from any "clear solution" of chlorides into a
dispersion colloid , a hydrosol , with loss of HCl and oxidation
to a dispersed form of probably metastannic acid . And the pH of that dispersion has great bearing on how well or poorly it adheres when it is baked
, what quality of coating
it produces .
[Edited on 26-1-2008 by Rosco Bodine] |
All solutions that I have been made using SnCl4 (solid) + Sb + HCl + Alcohol have remained clear. SnCl2 is another story.
Anyways I have moving away from the pathetic Shake and Bake (Sorry Eclectic) and going on to the lofty hights of spray pyrolylis as can be seen by the
exotic apparatus below.
It's a cillit Bang (or is it flash) sprayer with the spring removed and a string substituted. You must both push and squeeze to get it to work. It
will make mass production a reality...............
Dann2

|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It would have disappointed me if what I said didn't get a rise out of dann2  .
The chemistry is more complex than it might seem when SnCl4 is being used as part of a baked coatings precursor mixture , because it is unstable .
The chemistry is more complex than it might seem when SnCl4 is being used as part of a baked coatings precursor mixture , because it is unstable
both in the pot and on the substrate , it's composition is changing by the minute from when it is made . It is
so variable in composition and behavior that it produces
inconsistent and unrepeatable results unless you are
monitoring the variables in real time and following some
prescribed plan which realizes and specifies those variables .
The way to get past those variables is to use higher precursors for the SnO2 or indeed used the SnO2 itself
in the form of a stannic oxide sol or a stannate . Those
and the nitrate already contain the oxygen of SnO2 so
they don't have to lose chlorine and go looking for oxygen
from the air , as they brought their O2 lunch with them .
A hydrosol only has to lose water for example to leave an SnO2 layer , and there's no HCl coming off it to eat at any
spinel interface which it may be intended to seal , rather than to compromise .
As for SnCl2 not being used , well that's simply not true , but I'm not going back through a hundred references looking for the examples where it was
used just to prove it . But now that you have brought it up , SnCl2 is a fine precursor for
the kinder gentler oxidative soak deposition method which
I have recommended as an easy no brainer sort of method
of applying an SnO2 sealing layer onto a baked spinel interface . SnCl2 hydrolyzes and oxidizes simultaneously
to a hydrosol of SnO2 on exposure to air and this can alternately be done using dilute solutions and NaNO3 as the oxidant to deposit a tough film of
SnO2 onto a substrate ,
in a very slightly hydrated form which easily bakes out to
a tough and adherent film . Wait a minute , this isn't the way Diamond Shamrock said to do things is it ? You are right about that .....think of it
as a whole new state of the art .
[Edited on 26-1-2008 by Rosco Bodine]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by dann2
...... It will make mass production a reality...............
Dann2 |
Yes Dann2, all you need now is my 3 Kgs of assorted anode coating metals....
Hey!... What was that! Out the window! I thought I saw a pig flying past... 
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
dann2: My "SnCl4" solution remained clear, with a slight yellow tinge, when I added alcohol to it.
Rosco: I used excess H2O2, could this possibly extend the usable lifetime of the SnCl4 solution by keeping the oxidation state high?
I haven't inspected the new anode, I haven't decided how thick I want this coating to be. Probably will wait til morning, so I can have ~.5mm
thickness.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello.
| Quote: | Originally posted by Rosco Bodine
It would have disappointed me if what I said didn't get a rise out of dann2  .
The chemistry is more complex than it might seem when SnCl4 is being used as part of a baked coatings precursor mixture , because it is unstable .
The chemistry is more complex than it might seem when SnCl4 is being used as part of a baked coatings precursor mixture , because it is unstable
both in the pot and on the substrate , it's composition is changing by the minute from when it is made . It is
so variable in composition and behavior that it produces
inconsistent and unrepeatable results unless you are
monitoring the variables in real time and following some
prescribed plan which realizes and specifies those variables .
The way to get past those variables is to use higher precursors for the SnO2 or indeed used the SnO2 itself
in the form of a stannic oxide sol or a stannate . Those
and the nitrate already contain the oxygen of SnO2 so
they don't have to lose chlorine and go looking for oxygen
from the air , as they brought their O2 lunch with them .
A hydrosol only has to lose water for example to leave an SnO2 layer , and there's no HCl coming off it to eat at any
spinel interface which it may be intended to seal , rather than to compromise . |
Have you any examples of this being actioned in any patent, article etc etc.
All others may believe that the SnCl4 is not the way forward but I do not.
All patents, papers use SnCl4. None that I am aware of use other methods that you speak of.
Have read about Tin Sulphate on one patent and Oxalates in other patent (but Tin Oxalate as a pain to make)
Have you any pointers that show theses methods working for anodes, (ANODES) or intermediate coats for anodes (ANODES).
The only other conclusion I can come to is that there is a hugh coordinated and planned effort to by the scientific community to suppress the fact
that theses methods (that you speak of) work far far better than SnCl4 route.
Dann2
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
tentacles , see my edit above . You don't need SnCl4
for the sealing layer over the spinel , SnCl2 is just fine
since you are going from there to SnO2 using NaNO3 ,
if you want the easiest route .
@dann2
Trust me .
There are better uses for your SnCl4 .
It can be neutralized with ammonia to form
hydrated stannic acid , stannic hydroxide ,
which can then be neutralized with nitric acid
to form stannic nitrate , highly soluble and a
very much better candidate for a "dip and bake"
sort of coating .
Dissolved in excess ammonia it forms ammonum stannate ,
also highly soluble and when thickened a bit with PVA
or possibly with other things as well , it will also work
as a dip and bake .
Thirdly the SnCl4 can be used in making a mixed valency
Pytlewski polymer for use as a between baked coats wetting agent .
And antimony is a minor ingredient or not used at all ,
if all the references are correct in indicating that Bi is
the better dopant for SnO2 in DTO schemes .
[Edited on 26-1-2008 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@Tentacles
While I do not want to be 'running down' your efforts I suggest you test A DTO coat(s) if you want to see if you have a coating that could be
described as viable. You will have to put a coat on Ti alone as the Spinel will only confuse the DTO test.
Spinel coats as you are doing have been shown to be OK as per Xenoid and others.
The spinel alone with or without the (existant?) DTO may be fine as a percoat for the LD.
@at Rosco
No way!!!!!!!!!!!!!!!!!!!!!!!
Examples (or even a vague ref.) please.
Dann2
Waiting anxiously 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by dann2
Hello,
@Tentacles
While I do not want to be 'running down' your efforts I suggest you test A DTO coat(s) if you want to see if you have a coating that could be
described as viable. You will have to put a coat on Ti alone as the Spinel will only confuse the DTO test. |
Hmmmm. Pardon me but you don't know what you are talking about . These layers have different functions ,
but when they are baked diffusion occurs , so no one layer is a single quantity unto itself . That includes DTO onto a
titanium substrate where no spinel is the interface but a solid solution of the SnO2 and TiO-TiO2 suboxide . All the spinel does is produce a good
interface more easily and reliably than any chlorides concoction ever could . It's crisp and consistent in its formative reactions , not variable and
muddy and fickle and unpredictable .
| Quote: |
Spinel coats as you are doing have been shown to be OK as per Xenoid and others.
The spinel alone with or without the (existant?) DTO may be fine as a percoat for the LD. |
Nope it's going to need a sealing layer of DTO , just like I have said all along .
| Quote: |
@at Rosco
No way!!!!!!!!!!!!!!!!!!!!!!!
Examples (or even a vague ref.) please.
Dann2
Waiting anxiously  |
So schools back in session ....again ???? I'll swear you are
a stubborn hardhead about making the connection here .
But okay , I'll have at it one more time , I think this is three or maybe four ?? Anyway I lost track , but just for you dann2 
Oxidative soak deposition using SnCl2 , ( yes that is stannous chloride , the stuff you get using no peroxide , just good old HCl + Sn ) + KNO3 and a
little extra oxidation from limited air exposure . This produced a thin film deposit
of exceptional quality and uniformity , deposited on a polished glass substrate , which was so tough and adherent
that it could not be scraped from the surface being raked with the edge of a stainless steel knife . So the SnO2 is on there quite nicely from an
SnCl2 precursor . You argued with me about whether this would work on Ti substrate
ect. and no I don't have an article that shows it will stick
to spinel either , as it is simply a *deposition method* for
the SnO2 . Take my word for it , the SnO2 doesn't care
what it is precipitating onto and it will damn sure film onto anything and everything , particularly anything that is
hydrophyllic , such as anything having been treated with
a Pytlewski polymer in particular would be hydrophyllic , even if the substrate was teflon . In short , you can bet your ass
it will stick to Ti or spinel or any damn thing else . And when
it is baked , it's there forever when it sinters and diffuses .
And as to whether or not the thus originating coating has
usefulness as a coating for anodes , well the second attachment in the next post will clear that up , showing
definitely it does make an anode coating , with that coating
also derived from a stannous +II valency precursor as well ,
and a sol precursor derived therefrom , which is a variation
on the same oxidative process involved as described here in this attachment , the exception being that no additional oxidant other than air is used
for the second referenced method for arrival at the Sn +IV oxide .
Attachment: Preparation of SnO2 thin films by the oxidative-soak-coating method.pdf (324kB)
This file has been downloaded 1162 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
And here's the second SnCl2 related reference
I think there's more in the ATO thread concerning
the same topic , another article referencing SnCl2
as precursor in sol-gel deposition processes and I know there have been other examples referenced for Ti and
TiO2 substrates , even for depositions onto PET substrates .
You simply don't want to accept the valid scientific basis
for proposing there is a higher state of the art available
and published in the literature already , than what is
represented in some particular patent which you favor .
We are trying to go beyond what any one inadequate patent
has to offer and come up with something better .
At least that is what I have been doing anyway .
And I stand by the logical basis for what things I have submitted are pertinent to that task . I won't labor
with a deficient method , copying what somebody else has done , when I see the promise of a clearly better way ,
which shows no good reason evident why it should not work . Do you have some problem with suggestions
which may advance the state of the art ?????
Anyway , after you Oxidative soak deposit that first sealing layer of plain SnO2 onto the spinel and bake it , it will dope itself by diffusion from
the spinel below .
At this point the anode will be pretty well protected ,
and it's simply a matter of building thickness of coatings of whatever composition you like . Bi doped SnO2 would be
the type of coating to follow that seems to be the best
for sealing the substrate against oxygen diffusion which
ultimately leads to passivation . The Bi also has catalytic properties for perchlorate , so whatever Bi diffuses upwards
into any outer working coating such as MnO2 would be no problem .
Example 3 of US4272354 describes the use of SnCl4 +
Bi(NO3)3 as a baked coating precursor which forms a
working anode coating specific for chlorate and perchlorate
production .
[Edited on 27-1-2008 by Rosco Bodine]
Attachment: Sol–gel preparation and characterisation of mixed metal tin oxide thin films.pdf (428kB)
This file has been downloaded 4009 times
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hubert Update
Hubert and "Purple Haze" are still running well and producing KClO3 for me. Hubert has been running continuously for about 26 days (617 hours,
exactly) for a total of 1349 Ahrs. About a week ago, I dismantled Purple Haze, chilled it and extracted the KClO3. I replaced the small stirrer bar
with a bigger one and this keeps all the KClO3 in suspension. This seems to result in denser sand-sized crystallites forming. These can be scooped out
of the cell at about 3 - 4 day intervals, when the stirrer is switched off.
The colour/manganese dissolution is a bit problematical. I'm not at all sure what is going on. Purple Haze is actually colourless at the moment. When
I did the chilling-extraction a week ago the cell was slightly pink, it turned brownish and precipitated MnO2.H2O for a couple of days, now it is
clear. Needless to say it is losing Mn from the anode slowly, but the mechanism is anyone's guess. The period when Hubert was running at 3.6 amps (100
mA/cm^2) seemed to result in a loss of Mn and a marked deterioration in electrical parameters.
Hubert is now running at 4.0 volts / 2 amps versus 3.6 volts / 2 amps on the second day of operation. I think perhaps if I had kept the current
density at say 50 mA/cm^2 and the anode had not suffered during the electricity shutdown, it may have been capable of running for several months. I
guess now though, I'll let it run it for another week, then call it quits, I've collected about 600g of KClO3 so far.
Woah...! I'm now an International Hazard, better watch out ... 
[Edited on 26-1-2008 by Xenoid]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I am not argueing that you cannot deposit SnO2 films using Sol-gel, Hydrosol.
You can.
Theses methods have never been used for anodes. Do you know any refs. that show theses methods being uses as working anodes (ANODES) in (Per)Chlorate
cells? (or waste water treatment tests/applications or brine electrolysis).
First paper mentions nothing regarding anodes.
Second paper mentions anodes by referencing Anodes that are made by the Pyrolysis technique (not sol-gel, hydrosol) using SnCl4 + HCl + brushing on
with a soft brush + Pryolysis.
There is nothing to support your claims that I have seen or have been shown (apart from the large volume of argument that you are generating).
I wish anyone here who wants to try the Sol-gel etc methods the best of luck. Be aware that they have never been used or studied as (Per)Chlorate,
waste water, brine/Chlorine anodes. This is a fact.
These's methods are being put forward here as being superior, far better, etc etc to the SnCl4 +HCl + Pyrolysis route. Nobody anywhere has shown this
to be the case.
The SnCl4 + HCl + dip and bake + Pyrolysis route is being condemed as not workable ('dip and bake..... no way').
This is untrue.
No amount of words will change this fact.
The best way to educate your wee humble student of anodes is to show him some examples of your wise words of wisdom being actioned.
If there are no examples to be had, that is just the way the stuff works. ie. The sol-gel hydrosol etc does not work for anodes in
(Per)Chlorate/brine/Chlorine etc applications.
The dip/brush + SnCl4 + HCl + Pyrolysis does work and has been shown to work.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Deary me Rosco you are moving your posts around???????????
Hello,
Calm down.
Refs please.
Dann2
[Edited on 28-1-2008 by dann2]
|
|
|
| Pages:
1
2
3
4
5
6
..
13 |