YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Simple, but Little to no data? - reaction of sugars with alkali bases
looking up how to make calcium Gluconate I was reminded of something that bugged me for years.
when you mix household sugar (Sucrose) with NaOH soln, a reaction occurs leaving a thick clear viscous product that is no longer sweet like sugar.
this when left to dry will harden like rock.
What is it?
I was thinking about using Calcium Hydroxide instead, but I`ll wait to find out what the Sodium analog is 1`st, and then see if it`s viable as an
alternative to the gluconate.
I`d be happy just knowing it`s proper Name, as my searches have turned up nothing (all morning).
EbC: Title
[Edited on 23-1-2008 by chemoleo]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
[Edited on 21-1-2008 by Ozone]
Sonofab1C#!! "SUCRATE"
I had acknowledged nicodem's kindly note about the glucaric acid ( the diacid product of the oxidation of glucose, usually with dilute HNO3) salt
(Merck, 11th ED, pp. 1698) and added some information about the bastardization of the term "saccharate" by the industry. preview, edit post, and then,
nothing was left . .
Anyway, the alkali is not doing much to the sucrose. The sucrose is neither cleaving nor oxidizing under these conditions.The sucrose is likely
complexing the calcium hydroxide. from http://www.cheneylime.com/faqs.htm (because I am too lazy to re-type and reference the whole damn thing):
"When sugar is added, an intermediate product is formed, calcium sucrate (calcium hydroxide saccharate) which is significantly more soluble than
calcium hydroxide. For example; the addition of 35 grams of sugar will increase the solubility of the calcium hydroxide from 0.159 to 13.332 grams per
100 grams of saturated solution at 25°C"
Here the stoichiometry is Ca(OH)2:sucrose, ~1.76:1.
Reducing sugars are another story, and they will be present in all but the purest of sucrose preparations.
Thanks, LSD25, that was, more or less what I was going for when my post was hosed.
[Edited on 21-1-2008 by Ozone]
[Edited on 21-1-2008 by Ozone]
Attachment: www.epa.gov--ttn--chief--ap42--ch09--final--c9s10-1a.pdf (171kB)
This file has been downloaded 647 times
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
great, I`ll have a look into this in a sec, for Now I have something Really strange (to me anyway), basically I made the same strong sugar soln again,
you want it so that you will need to heat it in order to dissolve all the sugar crystals, OR just simply make a saturated soln with hot water.
I just did this and couldn`t believe my eyes when I added Calcium metal, it was Fizzing away just great but there was NO white turbidity or PPT!
I did the same again with plain water (same vol approx) with the same batch of metal, slow fizz.. rapid fizz.. BOIL with a white overflowing PPT out
the test tube. so my Calcium checks out just fine.
so I`ve now got another chunk in this strong sugar water and there still no PPT Nor is it boiling over even though it`s now quite viscous after the
1`st lot of Ca metal.
yes, by all means try this, it`s very simple and uses easy to get chems and a test tube 
as for the amount of Alkali metal/hydroxide added I just wing it, there`s no right amount really, when it goes Viscous and thick, you have the right
amount, as that`s showing the RXN.
the Calcium version I just tried gives a better idea that a RXN is taking place else where does the white Hydroxide go? 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
How concentrated is the sugar solution? Above ~60 g/100g the water activity (free water) drops like a rock (and, so, I suppose, the rate of rxn of
your Ca°). The Ca(OH)2 formed in-situ likely complexes quickly with the large excess of sucrose. The sucrate is soluble.
Dang, I have sodium, but I'll have to acquire Ca metal. The hydroxides, I have.
Interesting approach!
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
as for the soln concentrate, all I did was was put some sugar in a beaker and added boiling water to cover it by the same height again and stirred
well, not all dissolves, but IF per chance it does, just add some more until it doesn`t. there`s no need to keep it hot either.
then the liquid phase is decanted off into the test tubes.
NaOH gets added to one and Ca metal added to the other, I used 5ml per test tube and a single calcium granule roughly 4mm dia, and for the NAOH,
roughly add .25g.
both will dissolve nicely, and you notice the viscosity alters.
update, this 5ml soln has now used another ~4mm dia Ca granule and is still clear!
both are roughly 1/3 the viscosity of Glycerol.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
OK, it looks like your sucrose is in saturated solution (at rt, this is stable to at least 67 g/100g). Since there is not spontaneous nucleation, you
are not supersaturated (if your samples return to rt before adding the metal). At rt, this solution should be viscous (syrup) and the viscosity
increases over this when the metal is added.
I would expect a sharper viscosity gradient (if not out-right gelation) with Ca than with Na, are these different?
Check out "sucro-gels" which are documented by Willem Kampen, IIRC.
I'll see to this more a bit after lunch!
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
well to My Observations, the Ca version is slightly Less viscous than the NaOH version.
and BTW, it`s taking in it`s 3`rd Ca granule now! still no PPT???
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by YT2095
when you mix household sugar (Sucrose) with NaOH soln, a reaction occurs leaving a thick clear viscous product that is no longer sweet like sugar.
this when left to dry will harden like rock. |
I really don't see how any reaction could occur between sucrose and NaOH in water at such mild conditions. Utmost there is some proton transfer
equilibrium, but no real reaction. What makes you believe a product formed at all? Up to now you provided no such indication. Well, except in that the
NaOH treated sucrose lost the sweetness. Maybe you just did not neutralize it enough and perhaps sucrose is less sweet at basic pH?
Gluconic acid is prepared by the oxidation of glucose. Apparently hydrogen peroxide can be used which makes it easy to remove the side products from
the oxidation reagent, but it still must be difficult to remove gluconic acid from other side products of glucose oxidation. Perhaps you can
precipitate it from the reaction mixture in the form of the less soluble calcium gluconate by adding calcium acetate. That would be nice since that is
your target anyway. The problem is only in that the reference with the experimental is published in a hard to get paper. Check also the patents –
there are plenty describing the preparation of gluconic acid and calcium gluconate (though mainly by fermentation or electrochemical oxidations).
PS: What is it that you wish to achieve in dissolving Ca in an aq. solution of sucrose?
Attachment: gluconic acid (Ullmann).pdf (133kB)
This file has been downloaded 3118 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
for a start I can`t see how or what reaction occurs either, have you Tried this yourself?
as for the Calcium with sucrose soln, I find the result strange as in Not what I expected, of course the Next thing to try will be to Crystalise this
liquid and examine the crystals after (if in fact it Will crystalise).
I wish to achieve Insight as to what is happening in this whole reaction, this after all the Goal behind this site?
as for the Gluconate, although taking a back seat in this thread, I`m currently making calcium acetate on the strength of your advice, I am however
curious that Since this sugar calcium product is A) very east to make B) is nontoxic just like the gluconate and C) contains plenty of calcium as well
as high solubility, IF it would make a good replacement for the Gluconate for HF burns?
update, the Ca Sucrose soln has Also lost it`s sweetness too?
[Edited on 21-1-2008 by YT2095]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by YT2095
I wish to achieve Insight as to what is happening in this whole reaction, this after all the Goal behind this site? |
OK, I'll rephrase my question: What exactly you did and what kind of analysis you did for it to had you convinced a reaction occurred at all?
So, you are dissolving calcium in sucrose because you believe some precipitate would form and you think the precipitate would be calcium saccharate?
The problem is in that sucrose is not saccharic acid so there is no way for it to form calcium saccharate with calcium hydroxide. Also, Ozone made a
mistake in calling calcium saccharate a complex between calcium ions and sucrose. Calcium saccharate is simply one of the names for calcium glucarate,
the calcium salt of glucaric acid (also known as saccharic acid). Calcium saccharate (calcium glucarate) is available in health food stores.
Always check the literature first!
Calcium D-saccharate tetrahydrate:
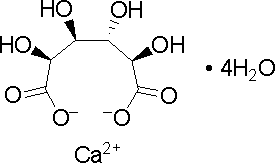
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by Nicodem
What exactly you did and what kind of analysis you did for it to had you convinced a reaction occurred at all?
So, you are dissolving calcium in sucrose because you believe some precipitate would form and you think the precipitate would be calcium saccharate?
|
no no no, I expected a CaOH PPT, and I didn`t get that at all, that`s why I checked my calcium metal in plain water just to make sure it WAS in fact
Calcium metal.
what I didn`t expect was to have No ppt at all, ergo there Must be Some reaction taking place.
that coupled with my new data that it is No longer sickly sweet but rather Slight sweet and very "dry" (by "Dry" I mean in the way of wine like
chateauneuf de pape).
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Originally posted by YT2095what I didn`t expect was to have No ppt at all, ergo there Must be Some reaction taking place.
|
OK, but you would have to compare the solubility of Ca(OH)2 in saturated aq. sucrose solution in order to say that is an indication that the Ca(OH)2
did not precipitate because of having been dissolved by a reaction product. A comparison with Ca(OH)2 solubility in water makes no sense as you are
not dissolving your calcium in water but saturated aq. sucrose instead (in which it would be reasonable to expect a higher solubility).
Try the same experiment in glycerol/water (10:1). Try dissolving Ca granules in it until you get a precipitate and compare with the amount needed to
form a Ca(OH)2 precipitate in pure water.
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
Sorry Nicodem, but I found this while looking up calcium saccharate:
http://tinyurl.com/ysny8m
It details the preparation and precipitation (using alcohol or cold water) of the 4 calcium saccharates using sugar (surely this is sucrose) and milk
of lime or plain lime. I presume they mean calcium oxide and calcium hydroxide?
Whhhoooppps, that sure didn't work
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Glycerol 10, water 1, and then add Ca.
ok dokes, I`m on it, I`ll also try the Ca(OH)2 solubility directly in Sucrose soln.
just an Idea, I was wondering sodium ethoxide, and since Sucrose is covered on O-H bonds, I can see how Sodium could be in place of a Hydrogen and not
really alter much on the Oxygen and it`s 2 lone pairs.
the Calcium however....
lets just say I`m still trying to work that one out 
Edited to add:
that has now been done, the Ca(OH)2 does Not seem to dissolve well in sucrose sol even when heated.
the Ca metal in 10:1 Glycerol/Water hardly reacts at all (although adding that water to it did make it warn up a little???).
I made another Glycerol/water soln 1:4 and there is a reaction, but the few bubbles are rising like in Honey, this May take a while!
[Edited on 21-1-2008 by YT2095]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by LSD25
Sorry Nicodem, but I found this while looking up calcium saccharate:
http://tinyurl.com/ysny8m
It details the preparation and precipitation (using alcohol or cold water) of the 4 calcium saccharates using sugar (surely this is sucrose) and milk
of lime or plain lime. I presume they mean calcium oxide and calcium hydroxide? |
That is actually quite interesting. Apparently the term 'calcium saccharate' in the year 1909 was used for a completely different complex/salt as it
is used today. Besides showing why (ab)using trivial names is not always a good idea, this old book also answers to your question on why there is no
Ca(OH)2 precipitate formed in your sucrose solution. Ozone was after all correct in saying that there exist complexes between calcium hydroxide and
sucrose.
I wonder if it is a property of all polyols to solvate calcium ions or is it just the cyclic forms of the saccharides that can do that. If it is just
the chelation with the neighboring alcohol groups, then Ca(OH)2 should be better soluble in glycerol than it is in water, hence the suggestion about
trying the same in diluted glycerol.
By the way, it is normal that Ca dissolves much slower in glycerol as it does in water. You can warm it up a little bit to speed up the reaction.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
well, after Much time, I came into the lab this morning and found that half of the Ca metal I left in the Glycerol was still there, it`s also a clear
soln still without turbidity other than that created by tiny bubbles.
interestingly and most notable is that the Ca metal in the Sucrose (after it`s 5`th granule) has started leaving crystals now, not a powder PPT, but
white Crystals, these crystals carbonise on heating too, even in soln.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks, Nicodem.
YT, can you post a picture of the crystals? I'd like to hear more about them. Titrate with HCl, perhaps? Solubility? I have got to get some Ca
metal . I have not seen this occur with the Ca(OH)2 (or direct from CaO)...ever.
Has the Ca metal:sucrose bit ever been tried before? I cannot find anything in the superficial literature. Maybe Beilstein if I have the time (a lot
of time)? Nothing in Vogel... . I have not seen this occur with the Ca(OH)2 (or direct from CaO)...ever.
Has the Ca metal:sucrose bit ever been tried before? I cannot find anything in the superficial literature. Maybe Beilstein if I have the time (a lot
of time)? Nothing in Vogel...
Ah, a little something (not with the metal, unfortunately) from Pigman, W. (1957). The carbohydrates. Academic Press, Inc. LCCC:57-8379, pp. 504:
Re. beet processing--"Additional quantities of sucrose may be obtained from the molasses by diluting it to a concentration of about 7% sugar, cooling
to 12°C, and adding lime (Steffen process). A difficultly soluble compound of sucrose withe three moles of lime, tricalcium saccharate,
crystallizes."
I too am curious about this. I think (speculation!) that the carbonyl function helps out quite a bit--with proper tautomerization (with a wee bit of
acid or alkali) it seems feasible that the sugar can play the enol-complex (aka. 2,4-pentanedione, six-membered "ring") game. I can rationalize (not
necessarily right or wrong) that this will not be as easy with glycerol without a little oxidation, first. Your guess is as good as mine.
Of further interest is that the solubility of CaO (and slaked lime) decreases as temperature is increased. This is why many sugar producers are using
hot-liming today (see Eggleston, G.)--adding the lime to hot juice means less soluble lime is needed; this equates to a decrease in scaling on heat
transfer surfaces (and the associated costs involved with wasted steam and time/money involved with cleaning). Whatever reaction is taking place,
however, between the solvated Ca(OH)2 and the sucrose will definitely go faster (Arrhenius kinetics apply) if heated (but, the overall rate will be
limited by the rate of solvation of further Ca(OH)2 at higher temperatures).
A little off-topic, but I thought that this was an interesting application:
Isolation of chitosan from chitin via alkali process and removal of subsequent calcium hydroxide via solution in 10% sucrose. I'll need to try this
(we have loads of crawfish and crab shells here).
http://xrint.com/patents/us/3862122
Cheers,
O3
[Edited on 22-1-2008 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by Ozone
YT, can you post a picture of the crystals? I'd like to hear more about them. |
I will TRY, but I doubt that my Cam is up to the task, unlike say Woelens photography that would show excellent pics.
I will Try however and as I type I have some of the liquid crytalising in a petri dish.
oddly the ones I`m doing that way are quite clear for the most part also 
I have considered the addition of an acid, but not HCl, I was thinking Dil H2SO4, that I expect will rip the Ca clean off to form the near insoluble
sulphate and maybe give me my sugar back?
I cant say what it will do until I try it 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
dangator
Harmless

Posts: 8
Registered: 22-1-2008
Member Is Offline
Mood: No Mood
|
|
Here you are
As noted above, reaction is unlikely with mild conditions. The paper gives products after refluxing with 50% NaOH. See table 1.
Attachment: sucrosedegradation.pdf (198kB)
This file has been downloaded 1559 times
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks, Dangator, that's a nice little reference (with a concise conclusion).
I only wish, however, that they would have quantitated the sugars over the course of the reaction. For sucrose and invert. This is easily done (if
expensive) with HPLC using an Aminex hpx-87K column and a differential refractive index detector. Kinetics (or lack thereof) would have been nice.
Cheers,
O3
[Edited on 25-1-2008 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by Ozone
YT, can you post a picture of the crystals? |
yup, here`s some from a batch of the Calcium Sucrate I crystalised slowly, there`s a really nice large crystal on the left.

all being well I should be getting some Barium metal today or tomorrow, and so I`ll try find out what happens using that instead.
I did a simple pyro test by making a mixture of this calcium sucrate with ammonium perchlorate, it burns Very well and with a very vivid red flame, as
good as Strontium anyday.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|