Difference between revisions of "TATB"
(→Chemical) |
|||
| Line 7: | Line 7: | ||
| OtherNames = sym-Triaminotrinitrobenzene | | OtherNames = sym-Triaminotrinitrobenzene | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Triaminotrinitrobenzene TATB structure.png |
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| + | | ImageCaption = Structure of TATB | ||
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
Latest revision as of 22:08, 26 October 2020
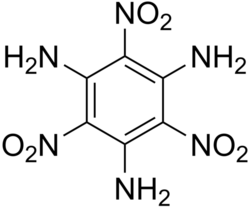 Structure of TATB
| |
| Names | |
|---|---|
| IUPAC name
1,3,5-triamino-2,4,6-trinitrobenzene
| |
| Other names
sym-Triaminotrinitrobenzene
| |
| Properties | |
| C6H6N6O6 C6(NH2)3(NO2)3 | |
| Molar mass | 258.15 g/mol |
| Appearance | Yellow or brownish solid |
| Odor | Odorless |
| Density | 1.93 g/cm3 (20 °C) |
| Melting point | 350 °C (662 °F; 623 K) |
| Boiling point | Decomposes |
| Insoluble | |
| Solubility | Soluble in aniline, chlorosulfuric acid, fluorosulfuric acid, nitrobenzene, conc. sulfuric acid, triflic acid Slightly soluble in DMSO Insoluble in glacial acetic acid, alcohols, benzene, chloroform, diethyl ether |
| Hazards | |
| Safety data sheet | None |
| Related compounds | |
| Related compounds
|
Trinitrotoluene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triaminotrinitrobenzene (TATB) or 2,4,6-triamino-1,3,5- trinitrobenzene is an aromatic powerful high explosive, based on the basic six-carbon benzene ring structure with three nitro functional groups (NO2) and three amine (NH2) groups attached, alternating around the ring.
Contents
Properties
Chemical
TATB is not very reactive, and doesn't react with glacial acetic acid, acetyl chloride, or acetic anhydride, even when heated to 150 °C in sealed ampoules.[1]
Decomposition of TATB releases carbon oxides, water vapors, nitrogen and lots of black smoke.
- C6H6N6O6 → 3 CO + 3 C + 3 H2O + 3 N2
Physical
TATB is a yellow solid, sometimes brownish depending on its form, insoluble in water and most common solvents, but more soluble in conc. sulfuric acid, aniline. Other solvents in which TATB is soluble are chlorosulfuric acid, fluorosulfuric acid, triflic acid (trifluoromethanesulfuric acid), molten diphenyl ether.[2]
TATB is yellow at standard conditions, but when exposed to UV radiation or gamma radiation from 60Co, it turns to shades of green. Studies done 1961 at Los Alamos revealed that the green color of TATB was caused by formation of an electronically excited state rather than by a new chemical species. The color varied from yellow-green to deep green to nearly black or brown-green.[3]
If heated to high temperatures, TATB turns dark yellow-brown.
Explosive
TATB is a powerful explosive (somewhat less powerful than RDX, but more than TNT), but it is extremely insensitive to shock, vibration, fire, or impact. At a pressed density of 1.80, TATB has a velocity of detonation of 7,350 meters per second and its RE factor is 1.17.
TATB is normally used as the explosive ingredient in plastic bonded explosive compositions, such as PBX-9502, LX-17-0, and PBX-9503 (with 15% HMX).
Availability
TATB is not available to the general public.
Preparation
TATB is produced by nitration of 1,3,5-trichlorobenzene to 1,3,5-trichloro-2,4,6-trinitrobenzene, then the chlorine atoms are substituted with amine groups.
Another route involves nitration and transamination of phloroglucinol.
A less known process described in literature involves the following steps: 1,1,1-trimethylhydrazinium iodide (TMHI) is formed from the rocket fuel unsymmetrical dimethylhydrazine (UDMH) and methyl iodide, and acts as a vicarious nucleophilic substitution (VNS) amination reagent. When Picramide, which is easily produced from Explosive D, is reacted with TMHI it is aminated to TATB.
Projects
- Make blasting charge
- Make benzenehexamine
Handling
Safety
Like nitroaromatic compounds, TATB is harmful if ingested and will stain the skin yellow.
Storage
In closed bottles.
Disposal
Can be destroyed by burning it in an incinerator. Due to its high stability and insensitivity, it's extremely difficult to accidentally detonate it.
References
- ↑ https://fas.org/sgp/othergov/doe/lanl/lib-www/la-pubs/00353872.pdf
- ↑ https://fas.org/sgp/othergov/doe/lanl/lib-www/la-pubs/00353872.pdf
- ↑ Cady, LANL, 1961