Difference between revisions of "RDX"
| Line 7: | Line 7: | ||
| OtherNames = 1,3,5-Trinitro-1,3,5-triazacyclohexane, 1,3,5-Trinitrohexahydro-s-triazine, Cyclotrimethylenetrinitramine, cyclonite, Hexahydro-1,3,5-trinitro-s-triazine, hexogen, T4, Trimethylenetrinitramine | | OtherNames = 1,3,5-Trinitro-1,3,5-triazacyclohexane, 1,3,5-Trinitrohexahydro-s-triazine, Cyclotrimethylenetrinitramine, cyclonite, Hexahydro-1,3,5-trinitro-s-triazine, hexogen, T4, Trimethylenetrinitramine | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = RDX structure.png |
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| + | | ImageCaption = Structure of RDX | ||
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
| Line 63: | Line 64: | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = 0.00597 g/100 ml (at 25 °C)<ref>Yalkowsky, S.H., He, Yan, Jain, P. Handbook of Aqueous Solubility Data Second Edition. CRC Press, Boca Raton, FL 2010, p. 61</ref> |
| SolubleOther = Soluble in dimethylacetamide, [[dimethylformamide]], [[dimethyl sulfoxide|DMSO]], [[N-Methyl-2-pyrrolidone|NMP]], hot [[aniline]], [[phenol]], warm [[nitric acid]]<br>Slightly soluble in glacial [[acetic acid]], [[acetone]], [[cyclohexanone]], [[diethyl ether]], [[ethyl acetate]], [[methanol]], <br>Insoluble in [[carbon tetrachloride]], [[carbon disulfide]], [[ethanol]] | | SolubleOther = Soluble in dimethylacetamide, [[dimethylformamide]], [[dimethyl sulfoxide|DMSO]], [[N-Methyl-2-pyrrolidone|NMP]], hot [[aniline]], [[phenol]], warm [[nitric acid]]<br>Slightly soluble in glacial [[acetic acid]], [[acetone]], [[cyclohexanone]], [[diethyl ether]], [[ethyl acetate]], [[methanol]], <br>Insoluble in [[carbon tetrachloride]], [[carbon disulfide]], [[ethanol]] | ||
| Solubility1 = 4 g/100 ml | | Solubility1 = 4 g/100 ml | ||
Latest revision as of 21:55, 26 October 2020
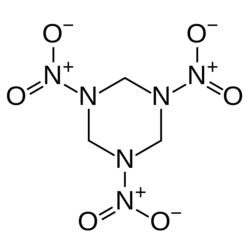 Structure of RDX
| |
| Names | |
|---|---|
| IUPAC name
1,3,5-Trinitroperhydro-1,3,5-triazine
| |
| Other names
1,3,5-Trinitro-1,3,5-triazacyclohexane, 1,3,5-Trinitrohexahydro-s-triazine, Cyclotrimethylenetrinitramine, cyclonite, Hexahydro-1,3,5-trinitro-s-triazine, hexogen, T4, Trimethylenetrinitramine
| |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C3H6N6O6 | |
| Molar mass | 222.12 g/mol |
| Appearance | Colorless crystals |
| Density | 1.82 g/cm3 |
| Melting point | 205.5 °C (401.9 °F; 478.6 K) |
| Boiling point | 234 °C (453 °F; 507 K) |
| 0.00597 g/100 ml (at 25 °C)[1] | |
| Solubility | Soluble in dimethylacetamide, dimethylformamide, DMSO, NMP, hot aniline, phenol, warm nitric acid Slightly soluble in glacial acetic acid, acetone, cyclohexanone, diethyl ether, ethyl acetate, methanol, Insoluble in carbon tetrachloride, carbon disulfide, ethanol |
| Solubility in acetone | 4 g/100 ml |
| Vapor pressure | 4.10·10-9 mm Hg at 20 °C |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
83.8 kJ/mol |
| Hazards | |
| Safety data sheet | Austin |
| Related compounds | |
| Related compounds
|
Hexamine HMX |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
RDX, also known as cyclonite, cyclotrimethylenetrinitramine, hexogen, is a nitroamine high explosive widely used in military and sometimes industrial appliactions, due to its good properties and low toxicity. RDX has the chemical formula C3H6N6O6.
The term RDX is an abbreviation for Research Department EXplosive, though it also stands for Research Department Formula X, though some sources also mention Royal Demolition eXplosive.
Contents
Properties
Chemical
RDX decomposes when heated to release carbon dioxide, water vapor and nitrogen gases, as well as soot and carbon monoxide. When initiated with a blasting cap, it will explode.
Physical
RDX is a colorless crystalline solid insoluble in water and poorly soluble in most common solvents. It is however soluble in hot aniline as well as hot nitric acid and liquid phenol. RDX is soluble in solvents with high boiling point, such as dimethyl sulfoxide, dimethylformamide, N-methyl-2-pyrrolidone. It has a density of 1.82 g/cm3 at room temperature.
Explosive
RDX has low sensitivity to impact and friction. If ignited, it will burn but will not explode. It has a high detonation velocity of 8750 m/s and a RE factor of 1.60.
Availability
Like most high explosives, RDX is not sold by any chemical suppliers. Diluted solutions of RDX, however, are sometimes available for analytical chemistry, though this source is very uneconomical and ordering large amounts will draw attention from the autorithies.
Preparation
RDX can be prepared by nitrating hexamine in cold nitric acid.
Projects
- Make blasting charges
Handling
Safety
While somewhat less toxic than TNT, RDX is still toxic if ingested, and exposure is known to cause seizures. It has low to moderate toxicity and it's classified as a possible human carcinogen, though it's unclear if exposure may actually cause cancer.
Storage
RDX is best stored in closed containers, away from heat sources. Avoid storing it though, as it may draw the attention of the authorities.
Disposal
RDX can be neutralized by burning it outside. Detonation is not recommended.
Oxidation with Fenton's reagent is another method that can be used. For safety, you should add it as diluted solution, dropwise to prevent it from splashing.
A cited method of neutralizing RDX involves adding it in a hot aqueous solution containing sodium hydroxide and sodium tetraborate. When the pH of the solution increases above 9.7, the RDX is neutralized.[2]
References
- ↑ Yalkowsky, S.H., He, Yan, Jain, P. Handbook of Aqueous Solubility Data Second Edition. CRC Press, Boca Raton, FL 2010, p. 61
- ↑ Jacqueline Akhavan, The Chemistry of Explosives, 3rd Edition, 2011, p. 148
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Nitrogen compounds
- Nitroamines
- Energetic materials
- High explosives
- Secondary explosives
- Things that can kill you very quickly