Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Why fractionating columns should be heated and/or well insulated
This is partly to have my own thinking challenged, but may be thought provoking for EtOH distillers.
I am in the process of building a general purpose still http://www.sciencemadness.org/talk/viewthread.php?tid=69333#...
Assume for now that my still is equivalent to
pot = 1 theoretical plate plus two columns of 5 theoretical plates each stacked vertically.
Using one of the most distilled substances by SM members, ethanol/water, as a starting point for consideration,
with this rough graph that I sketched in paint
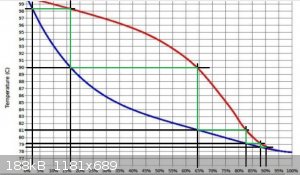
Suppose that we start with 50 litres of 2% EtOH boiling at 98 C
The first 'plate' will produce 5 litres of 16% at 90 C
The second will produce 1.56 litres of 64% at 81 C
Third, 1.22 litres of 82% at 79C
Fourth, 1.14 litres 88% at 78.5 C
...
...
Eleventh, almost azeotropic, c1.05 litres of 95+% at 78.2 C
My first observation is of the volumes involved;
in this case it would be best to do the first two (or more) distillations roughly
to reduce the volume that has to be processed by the fractionating still
... nothing new here.
My observation that may be of interest here is the temperature change from the fourth to the eleventh 'plates'
78.5 C to 78.2 C ..... a 0.3 C difference over 7 theoretical plates.
The implication being
if the upper seven 'plates' are not incredibly well insulated and a column heater is not used,
then the top seven 'plates' perform no useful function !
or, assuming a starting point of 20% EtOH w/w
if you do not insulate and/or heat your column
there is no point having more than three or four theoretical plates.
Does this seem correct, or am I missing something ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Sounds reasonable to me, that is why you never have more than two or three plates to start with and nobody goes from 2% to 96% in one go. It is
practically impossible.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Basically you need cooling to produce reflux. It can either be done by using an air-cooled column or a separate over head condenser. If an over head
condenser is used the column should be isolated. But as long as vapor is passing through the column you will get a temperature gradient, any heat loss
will simply add to the reflux.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
That is correct, but if you have more than two or three plates, the difference in temperature over the column past the third column becomes too big to
effectively separate (see the calculations in the first post). Practically you need insulation of the column instead of cooling when working with more
than one plate, because otherwise the passive cooling off the air already becomes strong enough to inhibit effective separation.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Sounds like you are on the right track. Temperature difference between the plates especially the top ones can occur just from the cooling effect of
the evaporating distillate.
No real need for active cooling. But it will be slow going, the rates of the product coming over are tiny with comparison to the amount returning to
the still. You've correctly identified that starting with 20%EtOH makes the target concentration easier to achieve and at higher rates. Adding a cold
finger to increase reflux will shift the equilibrium line to make a shallower gradient and therefore create the need for more stages.
Although you start with 20% EtOH this decreases because you are batch distilling, how much you have left in the still is related to how many plates
you have. Unless you are running a still with many tens of litres worrying about heating and cooling the column isn't necessary. A small 1-2 L still
with a decent column just needs the reflux rates slowly increased ( cold finger with a control tap) as the batch progresses. The PDF on distillation
theory with reflux rates I attached in the first thread (you linked in the first post) explains how to achieve a given composition with a column (I
hope. ) Was it of any use?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fulmen  | | Basically you need cooling to produce reflux. It can either be done by using an air-cooled column or a separate over head condenser. If an over head
condenser is used the column should be isolated. But as long as vapor is passing through the column you will get a temperature gradient, any heat loss
will simply add to the reflux. |
... is the 100 % correct answer.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The lighter component evaporates but the heavier component condenses. So the net effect is somewhat balanced.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Makes more sense that the column is cooled, otherwise the vapours have nothing to fight against.
If the column is Hot enough, everything would just stay in the vapour phase.
Controlling the pot temperature with a PID thing kinda throws a skew-ball into the mix, as most distillation calculations seem to assume a constant
heat input and a changing pot composition.
With PID control, the pot composition changes, but the pot temperature does not (significantly).
Seriously wierd to see a hardly-boiling pot and 66 C at the still head.
It would be nice to be able to see the vapours.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
In running an industrial column the primary consideration is product quality followed by production rate. For a lab I think it is the same with even
more emphasis on product quality. Heat to the boiler (pot) will be adjusted to meet the primary objectives. I don't think pot temperature is of
primary concern. In a batch process it will increase with time.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Blogfast: Thank you. While no expert I like to think I have a decent grasp of the principles involved.
Aga: That's the basics behind reflux. You need cooling, but you do have a choice in where it occurs. One is to cool the column, causing a continuous
condensation throughout the column. Or you can cool it above and return some of the condensate back into the top of the column. Both work just fine
(not sure about efficiency though), but an overhead condenser with adjustable reflux ratio will give more control over the process.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Chemetix
"The PDF on distillation theory with reflux rates I attached in the first thread (you linked in the first post) explains how to achieve a given
composition with a column (I hope. ) Was it of any use?"
Yes, it was/is useful, thank you
each new document has new information or a different perspective,
little snippets of information slowly coalescing into knowledge.
I often learn more from a second reading than the first,
once a mental framework develops it is easier to fit all of the pieces together,
and the significance of details missed earlier become apparent.
Great hobby !
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|