| Pages:
1
..
16
17
18
19
20
..
40 |
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
beautiful pictures, by the way, I have looked at each one and these are all quite pretty
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
It's long ago since I posted, here are the newest things(:
At first some azulene, what crystallized out from the reactions rest.

Second: mother of all column chromatographys. A 170cm tall column, 3000g of silica, 15liters of petrolether and 5 hours till the column did what it
should. Any comments? 

Check out my blog and see some other pictures(:
-and if You want to help me a bit, then please check out the google ads
CLICK
Edit by Nicodem: Changed the URL on author's request.
[Edited on 12/7/2012 by Nicodem]
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
That chromatography column . . .hmmm . . .the ultimatum of nerd porn?
I also like the huge glass funnels in the background and the crystal formations in the first picture are really nice....this sort of thing and crystal
growing/forming has to be one of the most enjoyable aspects of chemistry
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
There's some extreme tailing going on in that column there! Cool picture, but did it turn out okay?
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
That's an amazing chromatography column, it's taller than I am 
Did you make any TLCs to analyse the purity of your azulene?
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Yepp, TLC and also NMR.
The surpise was there was a black gunk what came down on the column with the azulene, but it didn't came down with the pure azulene(:
-it wasn't my reaction, it was just in that lab where I am also and I helped a bit.

I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Wow beautiful Azulene. I have always been fascinated after reading about it in my chemistry textbook and now seeing it is a reality. What procedure
did you use?
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
A similar one to what is published at orgsynth.org.
But the yields of the reaction wasn't so "awesome" as they reported...
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Recently I made some metals acetylacetonates!
-Cr(III) acetylacetonate

-Co(II) acetylacetonate

-Ni(II) acetylacetonate

I like very much their colors 
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Who's the poor intern who has to clean that column? lol
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Wow, I didn't know those salts were so beautifully coloured, ItalianChemist
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
I'm going to make other acetylacetonates!
I think that I made today: rubicene crystals

|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Make some acetylsalicycates (aspirinates?)!
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
I am going to post a red precipitate I'm making now.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Eddygp: why not simply post the picture when it is aleady ready?
Also, I have a few pics(:
Here, my actual fav picture:
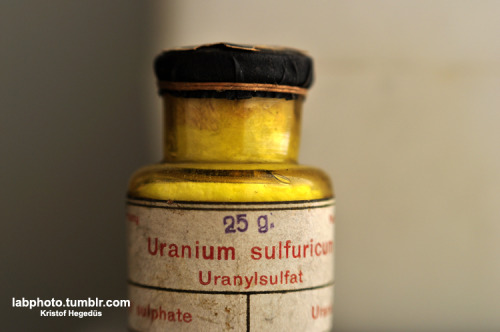
And another one made from some methyl orange and some crystal violet:

For more science realated pictures/videos, visit: http://labphoto.tumblr.com/ -and check out the google ads, thanks(:
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
On that last one: Now you're thinking with chemicals. XD
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kristofvagyok  | Eddygp: why not simply post the picture when it is aleady ready?
Also, I have a few pics(:
Here, my actual fav picture:

And another one made from some methyl orange and some crystal violet:

For more science realated pictures/videos, visit: http://labphoto.tumblr.com/ -and check out the google ads, thanks(: |
Please check your U2U.
|
|
|
virgilius1979
Harmless

Posts: 37
Registered: 30-9-2011
Member Is Offline
Mood: curious
|
|
Cu complex salts
Tetramine copper chlorate and Tetramine copper nitrate.


|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
[img]<a href="http://i1190.photobucket.com/albums/z444/scarface130/pic.jpg" target="_blank"><img
src="http://i1190.photobucket.com/albums/z444/scarface130/th_pic.jpg" border="0" alt="Photobucket" ></a>[/img]<a
href="http://i1190.photobucket.com/albums/z444/scarface130/IMAG0003.jpg" target="_blank"><img
src="http://i1190.photobucket.com/albums/z444/scarface130/th_IMAG0003.jpg" border="0" alt="Photobucket" ></a> all right! plante is showing me
how to upload pics. and i know gold plating is not chemistry and by far this not the prettiest picture but i wanted to show what i gold plated using
the cyanide process discussed in this forum. i got the salt and added water then put a gold anode/ gold cathode and let it electrolyze for about three
minutes before gold plating this stainless steel key chain. thanks plante1999
[Edited on 29-6-2012 by cyanureeves]
[Edited on 30-6-2012 by cyanureeves]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
virgillius' pictures are amazing! How can I make a tetraamminecopper ion?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Simply add ammonia to a solution of a copper (II) salt. You can easily isolate its salts. Look in Brauer in the library here for procedures.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i am getting a deep blue color by adding homemade ammonium chloride,baking soda and copper chloride crystals. i was trying to make smelling salts from
ammonium chloride and baking soda by heating the whole thing and scraping the glass wall for the salt. i happened to walk past a bunch of copper
chloride i had in a mayo jar and dumped it in as well.the blue liquid is nice and cool but the other jar with the rest of the copper chloride is
smoking after i added a couple of drops of ammonium chloride. im staring over with better reagents for a better purple color.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
Not sure if this counts as pretty, more like fascinating, but here's an outdated pic of my collection:

I made the table myself!
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
DoctorOfPhilosophy isnt that a wall shelving? how big are the pockets? color code each pocket and draw all the element symbols in them respectively
.neat and orderly is pretty as oppose to chaos unless chaos has bright colors.
|
|
|
adamsium
Hazard to Others
  
Posts: 180
Registered: 9-4-2012
Location: \ƚooɿ\
Member Is Offline
Mood: uprooting
|
|
Just thought I'd join in with a couple of pretty co-ordination compounds.
Firstly, some tetramminecopper(II) sulfate monohydrate

<hr>
Hexamminecobalt(III) chloride

<hr>
And here they are side by side

The yield of the hexamminecobalt (III) chloride was, as I recall, just under 0.5%, and this actually seemed somewhat better than many others achieved.
<hr>
Also, I tried to use the site's FTP rather than attaching the images. However, although I was able to connect, the directory was read-only. Do I need
to request FTP access? I read through the threads I could find on FTP and image uploads / insertion and couldn't find anything saying that any special
action was required.
If I'm able to get them uploaded properly via FTP, I'll edit this post or post a new one with those images rather than clunky thumbnails.
|
|
|
| Pages:
1
..
16
17
18
19
20
..
40 |