| Pages:
1
2
3
4
5 |
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
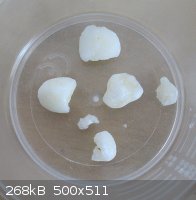
After cutting, washing in cold deionized water and drying with filter paper they are even less yellow now. Although they are far from crystals, I can
break them up between my fingers, and they definitely smell of HCl. They still dissolve into a small amount of water and leave a very clear solution,
except now, I can see how impure my tap water is, this stuff is more efficient than aluminium sulphate as a flocculant, you should see the
precipitates that this forces out of common tap water.
I will store in a dessicator with calcium chloride.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well done. The loss of colour is entirely expected: the ferric chloride is present only in SOLUTION, between the crystals. It doesn't crystallise out
because ferric chloride is highly soluble. By washing with pure water you've removed part of that solution, thus making the product whiter.
They smell of HCl because of the residual solution clinging to them. And trust me: they are crystalline alright, just not very pretty!
A very small amount of ferric chloride may have ended up trapped in the actual crystal lattice of your product but that's not really a problem.
Oh, and to effectively dry the stuff in a CaCl2 desiccator, crush the lumps to more or less the consistency of white granulated sugar: it will make it
easier for the remaining water to evaporate and become absorbed by the CaCl2.
[Edited on 3-6-2014 by blogfast25]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
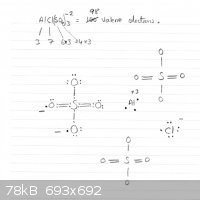
Ok then. And there I was trying to draw lewis dot diagrams of this to try and see something....(can't manage the lewis dot structure with this one,
others I have done but this one is difficult for me. Anyway, so ferric chloride makes sense. No I was expecting a spectacular array of sunshine
triangulating its way through crystal Planes of beauty. I still think they are too soft. Will try one more time later on and adjust ratios.
I have been trying for ages to work on this, Normally there are 2 Al ions needed to bond with 3 sulphates. Not having the second Al ion makes it
difficult especially since I can not see how on earth to solve this now with the chloride ion. It's now really annoying me since I solved so many
double and triple bonds and even a resonance question, I thought I could do this AGHRrrr...
Second question, why has the Al ion in soln a positive 3 charge when it misses 5 electrons from its octet requirement?
The more you learn the more you realize you don't know....and for every question answered three more questions rear their irritating little heads.....
[Edited on 3-6-2014 by CHRIS25]
[Edited on 3-6-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Chris:
You're making it more complicated than it is. No Lewis dots needed here. AlClSO4 is slightly unusual (with respect to Al sulphate) in the sense that
you have in this type of lattice 1 type of cation (Al) and two types of anion (1 sulphate, 1 chloride). It's uncommon but possible.
Al's electron configuration is [Ne] 3s<sup>2</sup> 3p<sup>1</sup>. When it 'loses' these three valence electrons
(3s<sup>2</sup> 3p<sup>1</sup> its electronic structure
becomes that of Neon. Simply put: Al<sup>3+</sup> = [Ne]. And the electron configuration of neon is a full octet. its electronic structure
becomes that of Neon. Simply put: Al<sup>3+</sup> = [Ne]. And the electron configuration of neon is a full octet.
The only other way Al could acquire an octet structure would be by absorbing 5 electrons, so it would become Al<sup>5-</sup> (!!!) but
that is thermodynamically totally unfavourable because of the mutual electrostatic repulsion of those electrons.
Re. softness, allow to dry, then reassess.
The more you learn the more you realise you know hardly ANYTHING, true for everyone (but few know it).
[Edited on 3-6-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | I think you saw and smelled HCl |
Yes. Very likely.
Bad choice of words on my part due to limited experience.
Nobody is born knowing anything at all.
The point where you think you know everything is precisely the point at which you stop learning.
@CHRIS25
Nice one.
I shall chill some water and see if my earwax also goes Ultrabrite.
[Edited on 3-6-2014 by aga]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Aga: I don't have the apparatus to do this, but I was reading a patent yesterday and noticed one paragraph. It gave me an idea, maybe you could
follow the procedure through to the point where you cool, but don't allow it to solidify, then pump HCl gas into the solution for a few minutes. then
allow to cool and solidify? I have no idea but maybe worth trying?
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CHRIS25  | | [...] then pump HCl gas into the solution for a few minutes. then allow to cool and solidify? I have no idea but maybe worth trying?
|
'Gassing' (as we call it) a concentrated solution of Al sulphate with HCl gas is an option I've considered already. But it means making a HCl
generator (which really isn't too difficult), it just complicates things a bit. More and a better product may result from it though. Definitely worth
trying... The advantage of gassing is that you don't add more water, this should at least give greater yield of product.
@aga:
You suffered from confirmation bias, I think. The smell of HCl, the yellow colour of the solution, possibly visible fumes of HCl... all these fooled
your associative brain into 'seeing' chlorine. It's a very common mistake.
[Edited on 3-6-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
@blogfast25
Being such a noob, i have never seen actual chlorine gas, so should not have sait it was chlorine.
All i really saw were wisps (3-4 inches) of steam-like stuff twisting out of the beaker, and smelt something clorine-y.
@CHRIS25
'gassing' seems like a Good plan - no added water, no boiling Off excess water.
As the reaction is with HCl, perhaps just Al Sulph (gawd bless 'im) and HCl gas will work much easier.
It sounds like it will be easier to produce a pure Gas than a pure solid or liquid.
Someone jump in and correct me if this is plain stupid.
Busy day tomorrow, so unlikely to get anywhere with it until Thursday.
Now to find out how HCl gas is produced, and how to survive doing so.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I dissolved 20 g of aluminium sulphate hydrate (ASH) in 20 ml of HCl 37 w% by simmering for a bit. I used commercial grade ASH (n = 14.3, MM = 600
g/mol, iron free).
On cooling the whole mass slowly crystallised, so that was no good.
I then added another 20 ml HCl and reheated to complete dissolution (the solid dissolved very quickly). On cooling I obtained a mass of snow white
small crystals (0.5 - 1 mm), at a guess about 10 g. They look very different from ASH: much more crystalline.
The supernatant liquid was also yellow because of iron in my HCl.
1 mol NaCl + 1 mol H2SO4:
NaCl(s) + H2SO4(l) === > HCl(g) + NaHSO4(s)
You don't even need to heat it but you do need 95 - 98 w% H2SO4.
This reaction works because hydrogen chloride is a gas and leaves the reaction. But water is the enemy here because HCl is so damn soluble in it, so
water will reduce yield of gaseous hydrogen chloride.
There are various threads on HCl generators on this forum. Search and ye shall find.
[Edited on 3-6-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Only found 3 so far ... 
Quick thought : why does the 'gassing' need to be done in a beaker ?
It doesn't does it ?
The surface area will be an important aspect - more = better/faster.
Al Sulph Hydrate (OldAl) when heated goes liquid.
So, if i make a box that is gas-tight, pump far too much HCL into it (purge the Air) and then Spray the OldAl solution in an aerosol Up into that
gas, then keep heating/pumping OldAl until it pumpeth no more, then the reaction should be complete.
I guess the product would be spattered all over the container, but it should be pretty pure.
Does that sound like madness ?
[Edited on 3-6-2014 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Erm... affirmative. Repeat: AFFIRMATIVE, roger.
Why go to that trouble if you can simply lead HCl gas into a beaker with saturated aluminium sulphate solution? The gas will dissolve in the solution
and on cooling (presumed) aluminium chloride sulphate should crystallise out. Or so says the plan.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. affirmative. We got a Green Light then.
Insane method versus Beaker & inverted glass funnel gas dispenser/anti-suck-back device.
Experimentation will tell us which works best.
No bets though.
Hang on.
Even if OldAl sticks to the sides Un-reacted, the presence of the gas will cause a reaction, so adding the liquid to the Gas in this way should work
out better, as in faster, seeing as the available surface area for the reactants to encounter each other will be many times higher than a bubble of
HCl.
Clearly the Gas concentration must remain high at all times, so just need to keep pumping it in.
Equally clearly, the hydrate required needs water.
Calculation required.
[Edited on 3-6-2014 by aga]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Be careful to avoid suck-back, the high solubility of HCl (g) will cause the solution to flow back into the gas generator.
A simple anti suck-back devise will contain any and all problems, should they occur/when they occur.
[EDIT], Ah, I see you already know about such things.
BTW, would chlorine work to make AlClSO4, quite a bit cheaper.
[Edited on 3-6-2014 by Zyklonb]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Such things yes, but not That thing !
What is it called, and where can i get one ?
I have no idea.
Will bubbling Chlorine thru OldAl make the same as bubbling HCl through it ?
[Edited on 3-6-2014 by aga]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
After looking for more than 5 minutes I couldn't find one anywhere except Alibaba:http://heqi.en.alibaba.com/product/256344917-200694327/anti_suck_back_bubbler.htmlhttp://heqi.en.alibaba.com/product/256344917-200694327/anti_suck_bac
k_bubbler.html
You have to buy only 100 pieces, you'll need that many right?
I don't have one, although it would be nice, a vacuum flask or similar will work just as well.
Quote: Originally posted by aga  |
Will bubbling Chlorine thru OldAl make the same as bubbling HCl through it ?
[Edited on 3-6-2014 by aga] |
Thats what I was wondering, I work with chlorine quite a bit, as long as you have a gas mask, it's a very pleasant substance to work with.
[Edited on 3-6-2014 by Zyklonb]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Blogfast: used 0.033 mol sulphate and an eventual 0.472 mol HCl. This is a 14:1 ratio; I used a 7:1 ratio. It seems more acid produces smaller
crystals then. Tomorrow, if not raining still, I will try midway between these two just for fun, and then call it a day.
ZyKlonb: That piece of apparatus does not look suitable anyway, major problem that I see is no way to get any solid out that may form inside. It
seems only suitable for gases and liquids.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's an experiment, so i guess the inverted funnel will be OK.
After all, this isn't Industrial Science Best Practice is it ?
Anyhoo, y'all jump in and do a simple experiment and show us noobs how it is done.
My bet is on my idea - squirt the OldAl soln into the gas.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | and then call it a day. |
Not a Chance.
Got to hammer it flat or it will forever be wobbly.
| Quote: | | Thats what I was wondering, I work with chlorine quite a bit |
If you work with Chlorine, then you are in a much better position to answer that question than most.
Assuming that the chlorine Ion is involved in the reaction, and already knowing that water is present, so suspect that Cl dives into the water and so
Cl ions happen.
Sequester a litre or 22.4 and try it, and tell us if it works.
Simples.
[Edited on 3-6-2014 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
Good luck!
@Zb:
Boy, is that piece overkill or is it overkill?  All you need is an empty
container with an unobstructed inlet (connected to the generator) and an unobstructed outlet (connected to the container you're gassing). In case of
suckback the liquid is sucked into the container. Been there, done that. The piece you show would be suitable for gassing something but even for that
purpose it's overkill. And what Chris said... All you need is an empty
container with an unobstructed inlet (connected to the generator) and an unobstructed outlet (connected to the container you're gassing). In case of
suckback the liquid is sucked into the container. Been there, done that. The piece you show would be suitable for gassing something but even for that
purpose it's overkill. And what Chris said...
BTW, it's not the solubility of HCl in water that can cause suckback. And with the inverted funnel method there can be no suckback anyway. But I still
prefer leading the gas directly into the solution to be gassed...
Chlorine won't work here: there's no way to reduce it to chloride without additional chemicals.
@Chris:
Careful drawing conclusions like that: how do you know it wasn't crystallisation temperature for instance that caused this more compact,
microcrystalline product?
But by all means try my ratio...
@All:
FORGET chlorine. There's no way it can react here to chloride and chloride is what we need here.
[Edited on 4-6-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I just had a quick go at the HCl gas idea.
First off i knocked up and tested the gas generator (used coffee jar+irrigation valves+lost of hot melt glue), and the reaction of NaCl +
H2SO4 seemed quite slow, so i add quite a lot of salt and controlled the addition of the acid.
Interestingly the HCl gas feels 'hot' on skin, wheras the actual reaction solution doesn't feel even warm through the glass.
Then 5g of Alumnium Sulphate (unknown hydrate) as added to a vacuum flask with no additional water and the tube attached.
After running the generator for 30 seconds, a Pressure Sensor was fitted to contain the gas in the flask, and verify gas production.
Almost immediately the white Aluminium Sulphate crystals turned yellow.
Afer 30 minutes the crystals are showing white spots, mostly at the edges, and appear 'wetter' than before, in that they leave traces on the glass as
the flask is swirled.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Ah right, I don't know how I missed that.
Al2(SO4)3 (aq) + 2 HCl (g) → 2 AlClSO4 + H2SO4, that works, but
Al2(SO4)3 (aq) + Cl2 (g) → 2 AlClSO4 + SO4-, there's no
cation to combine with the sulfate anion. [Face-palm]
Quote: Originally posted by aga  | Almost immediately the white Aluminium Sulphate crystals turned yellow.
After 30 minutes the crystals are showing white spots, mostly at the edges, and appear 'wetter' than before, in that they leave traces on the glass
as the flask is swirled.
|
Weird. Why is it always turning yellow, some kind of side reaction? Doesn't make much sense...
[Edited on 4-6-2014 by Zyklonb]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | So, if i make a box that is gas-tight, pump far too much HCL into it (purge the Air) and then Spray the OldAl solution in an aerosol Up into that
gas, then keep heating/pumping OldAl until it pumpeth no more, then the reaction should be complete.
I guess the product would be spattered all over the container, but it should be pretty pure.
Does that sound like madness ? |
This sounds terrifying and overcomplicated, to say the least. The inverted funnel trick should work perfectly here, considering HCl's high solubility.
It's also a lot less crazy, without pumps, boxes full of pressurized corrosive gas, and tedious scraping of contaminated product off the walls ala an
exploded microwave burrito.
In case it isn't entirely clear, here's a picture I dug up from the internet on how the inverted funnel trick works.
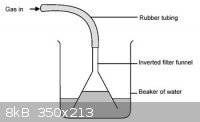
If suckback starts to occur, the liquid level outside the funnel falls until it breaks the seal and equalizes the pressure. In the case of very
soluble gases like HCl, you may not even need to immerse the funnel in the solution at all - just suspending it a few mm above (while stirring) may
work.
Edit: The rest of that page is actually pretty interesting. It's the classic ammonia fountain demonstration, except with HCl instead:
http://www.nuffieldfoundation.org/practical-chemistry/proper...
There's also a nice graphic of an HCl(g) generator:
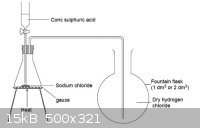
[Edited on 6-4-2014 by MrHomeScientist]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Yellow after all?
I am beginning to question the iron theory. there are hardly any images of this chemical to be found, here is the only one I could find:
http://www.feralco.com/ES/UK/page858-phal-10.php
Not perhaps best but this also:
http://www.lehighminerals.com/Z4auction339oct22.htm just scroll down to KLEINITE now I know there is mercury here but mercury yellow? No.
It does appear so far that just maybe our chlorosulphate should be yellow after all, and that yellow is provided by ???
I am not discounting the ferro idea, but where is Iron coming from in HCl gas, nowhere....
[Edited on 4-6-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I don't think the feralco product is the same as ours. The 'poly' I a bit of a giveaway. The feralco product is for water treatment: a bit of ferric
chloride in there would do no harm. It's also a technical grade: they probably started from technical HCl solution.
It also doesn't explain why my crystals are snow white and only the supernatant liquid is yellow. I will test it later on with ammonium thiocyanate.
Also, Al cations, sulphate ions and chloride ions are all perfectly colourless: hard to see how something made from these 'building blocks' would be
coloured...
What is very puzzling is that search terms like 'AlClSO4', 'aluminium chlorosulphate', 'aluminium chloride sulphate' yield almost nothing (except
really for the low authority 'atomistry' and NEVER a CAS number or similar known chemical identifier (EINECS and such like).
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | be yellow after all, and that yellow is provided by ???
Also, Al cations, sulphate ions and chloride ions are all perfectly colourless: hard to see how something made from these 'building blocks' would be
coloured...
What is very puzzling is that search terms like 'AlClSO4', 'aluminium chlorosulphate', 'aluminium chloride sulphate' yield almost nothing (except
really for the low authority 'atomistry' and NEVER a CAS number or similar known chemical identifier (EINECS and such like). |
Ok I do understand, but what about aluminium chloride most of the products are yellow?
You are right about the search, I have in between other things spent two days on and off trying to find anything, nothing at all, except a few
patents.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
| Pages:
1
2
3
4
5 |