| Pages:
1
2
3
4
5 |
Texium
Administrator
       
Posts: 4546
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by deltaH  | I used to think the same as you, but then I tried it...
The boiling does smell fishy because in the beginning, the trimethylamine that's initially in there (typical of commercial samples) boils off, but
later it doesn't smell fishy and the freshly crystallised crystals don't smell fishy at all either. It's only upon aging that the characteristic odour
develops again.
For all intent and purposes, the crystals are dry except for trace water. If you do it, you will see.
I think the viscous liquid is molten choline chloride. I didn't measure the temperature, but it was @#!%! hot at the end (gas burner on full with a
large skillet pan over the flame). Luckily I had a hunch that it was dry and so turned off the gas. I was pleasantly surprised when cooling that
everything solidified into a crystal mass.
My point... you CAN simply just boil it down, so long as you know where to stop it. Anyway, if you stop it too early and it's too 'wet' when cooled,
just turn up the heat and heat some more. |
Then how is it that when a few months ago I decided to try drying
some damp reagent grade ChCl using medium heat on a hotplate at my school, it burnt black within a few minutes and made the entire classroom smell
like a burning fish market? (to the despair of the AP chemistry class I was sharing the room with)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Because yours was probably near the end point of what I made. I stopped mine just before it got very hot and presumably would start decomposing. When
it cooled, the liquid solified into a slightly damp mass of white odourless crystals. If I had continued to heat this, it would likely have burnt too,
I simply acted on a gut feeling not to heat further. Turns out at that point most of the water was gone.
Due the extreme hygroscopic nature of ChCl, I would think that what you see as 'damp' crystals, is in fact a very small amount of total water (or
perhaps ethylene glycol). What I mean, let's say saturated ChCl is 80% solution (thumb-sucked) and let's say that your crystals are dampened by 5%
saturated solution, so the water causing the apparent dampness is just 20%*5% = 1% overall!
Finally, this IS very stinky! I did my boiling outside and slowly so as to limit the stink. No doubt some decomposition occurs in the process, but the
product was pure enough for my needs, white crystalline and odourless... at least for a while.
I made very nice choline soap with it, the only problem is that after a while, my soap bars went from being odourless to very fishy, which made me
really sad because it worked very well otherwise, i.e. it made a beautifully mild and gentle soap.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by DeIonizedPlasma  |
No, sadly choline chloride just likes to undergo a Hoffman degradation into trimethylamine (wonderful fishy smell), water, and ethenol/acetaldehyde
from what I can find. Mechanism picture attached, taken from Electrochemical decomposition of choline chloride based ionic liquid analogues. Given that it requires the hydroxide rather than the chloride, I
think it may be happening due to trace presence of water. I couldn't find more mechanisms on the degradation, but I wonder if there isn't one that
would occur in anhydrous ChCl. Perhaps someone should attempt to seal some choline chloride with negligible water content and leave it out for a while
to test if it produces trimethylamine.
[Edited on 9-12-2015 by DeIonizedPlasma] |
I noticed that the hydroxide degraded when preparing my soap. In an early experiment, I simply dissolved my NaOH directly into my 75% ChCl solution
letting it get hot as well. This was bad, it seemed to degrade rapidly and the solution turned yellow-orange!
I had the idea that removing water might prevent this, so I proceeded to dry my ChCl solution by the method described before, then dissolving the ChCl
into 1.5 mol. equivalents (off the top of my head, check the choline soap thread) 100% glycerine and only then added and blended the NaOH. This was
better behaved and didn't yellow much, at least in the time it took to make the soap.
Unfortunately, the soap still formed trimethylamine in time when used, possibly because it gets wet. Water might be speeding up the process.
[Edited on 9-12-2015 by deltaH]
|
|
|
Texium
|
Thread Topped
22-12-2015 at 08:02 |
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Trimethylglycine also decomposes to trimethylamine but not very much.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
To those interested in RTILs (Room Temperature Ionic Liquids), the following book has been published recently: "Ionic Liquid Properties - From Molten
Salts to RTILs"
http://link.springer.com/book/10.1007%2F978-3-319-30313-0
The last chapter is over 70 pages, and is all about RTILs.
I don't have full access, but maybe some mad scientists do.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Attachment: Ionic Liquid Properties_ From Molten Salts to RTILs.pdf (3.5MB)
This file has been downloaded 3811 times
|
|
|
zck1214
Harmless

Posts: 3
Registered: 8-9-2016
Member Is Offline
Mood: No Mood
|
|
I can not receive the eutectic solvent when using AlCl3 (anhydrous) and urea, Who can tell me why?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Magic elves?
Need more details from you. No one can give a meaningful answer with no information to go on. Tell us what exactly you did and where it went wrong.
|
|
|
zck1214
Harmless

Posts: 3
Registered: 8-9-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | Magic elves?
Need more details from you. No one can give a meaningful answer with no information to go on. Tell us what exactly you did and where it went wrong.
|
The mole ratio is 1:1, N2 protection and nothing happened at room temperature, so I increased to 120oC, but only white powder can be found. Thanks.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I'm just speculating, but urea doesn't melt until 130 C so you may not have heated it enough. Also ensure your aluminum chloride is really anhydrous;
a difficult task if not in a professional lab. A nitrogen blanket is a good idea.
When I made the urea / choline chloride liquid, I melted the whole contents of the test tube with a propane torch. No precise temperature control.
Maybe the AlCl<sub>3</sub> version behaves differently though.
|
|
|
zck1214
Harmless

Posts: 3
Registered: 8-9-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | I'm just speculating, but urea doesn't melt until 130 C so you may not have heated it enough. Also ensure your aluminum chloride is really anhydrous;
a difficult task if not in a professional lab. A nitrogen blanket is a good idea.
When I made the urea / choline chloride liquid, I melted the whole contents of the test tube with a propane torch. No precise temperature control.
Maybe the AlCl<sub>3</sub> version behaves differently though. |
Thank you. I have tried higher temperature than 130 C, the white smoke on the top of tube exhibits the sample evaporated. Maybe my AlCl3 has not high
purity, I will try again after vacuum drying. Thanks.
|
|
|
jktan26
Harmless

Posts: 1
Registered: 6-7-2017
Member Is Offline
Mood: No Mood
|
|
Type II DES (Choline Chloride with MgCl2.6H2O)
Has anyone able to synthesize type II DES ? I had tried every molar ratio according to journals ranges from 1:1 to 2:1 (MgCl2.6H2O:Choline Chloride).
It seems like the products i got are always in milkish color (as shown in the attached photo) even though they are in liquid form at room temperature.
Any idea to resolve this? 

|
|
|
yobbo II
National Hazard
   
Posts: 719
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
An eutectic, melting at 310C, in the system sodium perchlorate-barium
perchlorate occurs at 43 mole per cent barium perchlorate.
Zinov'ev, A. A., Cludinova, L. I., and Smolina, L. P., Zhur. Neorg. Khim., 1, 1850
(1956).
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I have experimented a bit with DES, in particular type 4 in my search for a method for electrodepositing chromium from Cr3+ salts (and also cobalt,
though I have a simple system for that). I attempted to make DES by melting together CoCl2 and CrCl3 respectively with urea in equimolar amounts. The
resulting liquids are very viscous, almost unmanageably so. They also solidify in mass at room temperature after a few hours. While they were still
liquid I attempted electrolysis in order to deposit the respective metals onto cathodes of copper and nickel, with anodes of Co and Cr respectively.
Not much success, some thin deposition occurred but the deposits were thin, non-adherent and dull.
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I've been thinking a bit more of revisiting DES and chromium deposition since I last stumbled on this thread and posted above. I found a PhD thesis
from U. Leicester https://lra.le.ac.uk/bitstream/2381/38111/1/2014AlbarzinjyAA... which investigated this exact matter. After reading through it, it looks that they
had the best results with non-eutectic mixtures (non-eutectic eutectics, is that a thing?) and additives.
The best results were with a 2:1 molar mix of urea:CrCl3.6H2O with addition of 20% w/w free water and 0.1M boric acid. The next best was with 2:1
molar mix of urea:KCr(SO4)2.12H2O with the same additives (20% w/w water and 0.1M boric acid). Deposition done on mild steel substrates at 40C for 1h,
current density 150mA/cm^2 for the first and 130mA/cm^2 for the second. They are both reported to have much lower viscosity than the pure DES, better
conductivity and produce bright chrome layers with good thickness and hardness.
I will try to reproduce that if I can. I don't have CrCl3, but I do have some chrome alum. They mention some other additives such as benzoquinone (not
sure if 1,2 or 1,4) but they haven't been tested in that paper. Still searching for more data on that, may try later on.
Will report back with results when available.
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
No results yet, but some pretty pictures with color changes.
I made the type 4 DES today on a 1/10 mol scale. Molar ratio 1:2 KCr(SO4)2:urea is 499g:120g, or at 1/10 scale 49.9g:12g
To this will add 20% water and boric acid to 0.1M. Per calculations that means 12.7g of a 2.5% boric acid solution that will bring both the required
water and the boric acid.
Pictures below.

Chrome alum purple at the bottom, urea white on top.

1 minute in microwave, it started melting and changing color.

Another minute in microwave, it became really hot and it almost completely melted. Some alum crystals were stubborn. Nice emerald color. Consistency
of syrup.

After cooling down a few minutes, in natural light. It became much thicker, like honey. Too bad I can't show the consistency in pictures.

After adding the boric acid solution and stirring for a few minutes. All dissolved. Same color. Much less viscous, similar to molten ice-cream.
I'll let it rest for a day or two (principally because I lack time to continue now) then onto electrolysis.
[Edited on 29-7-2018 by stamasd]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
For those ionic liquids that contain choline hydrochloride, is there a problem with the liquid absorbing water from the atmosphere?
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I don't know. So far I've only experimented with types of DES that don't use choline (type 4). In fact until today I didn't even have choline
chloride... and then the mail came with my bag of choline. 
Urea is also slightly hygroscopic but not deliquescent. With the type 4 using urea I have noticed however somewhat the opposite problem. I think that
if exposed to air, some of the water that is brought in as crystallization water of the alum evaporates, and that is one of the factors that leads
eventually to the solidification of the DES. I have noticed that a previous sample of a similar DES to the one above (that one was made with 1:1 alum
to urea) after less than a day had become very viscous at the surface, almost making a "skin" at the top. And then it started becoming a solid mass
from the top down. That's why I think there was evaporation at play.
[Edited on 30-7-2018 by stamasd]
|
|
|
BaFuxa
Hazard to Self
 
Posts: 61
Registered: 18-9-2017
Location: Mars
Member Is Offline
Mood: Buzzing
|
|
Note on Type IV DES using urea as hydrogen bond donor : the urea decomposes fairly quickly. Once I used a MgCl2-Urea DES and everything started
smelling ammonia when I heated it up to about 150-200°C.
[Edited on 24-10-2018 by BaFuxa]
Potential counts for nothing until realized.
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
Quote: Originally posted by BaFuxa  | Note on Type IV DES using urea as hydrogen bond donor : the urea decomposes fairly quickly. Once I used a MgCl2-Urea DES and everything started
smelling ammonia when I heated it up to about 150-200°C.
|
FWIW there was no ammonia smell while making the DES above (urea-chrome alum) nor since. Perhaps it's a better idea not to heat urea-containing
solutions until it decomposes. 
|
|
|
j_sum1
|
Thread Split
14-1-2019 at 15:20 |
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
A little (belated) update on my chrome alum/urea/boric acid/water liquid above. I stuffed it in a drawer and it stayed there for a few months. I did
get around yesterday and test it in an electrolysis experiment. Using a stainless steel anode, a copper cathode, electrode area about 1cm^2 each, 10ml
electrolyte, electrode distance average 2cm, adjustable power supply.
First observation, it does conduct electricity but much less than a saline/water solution. To achieve a current 0.1A I had to crank the voltage to
about 9V. That's an impedance of 90 ohms, vs 5-10 ohms for a similar saline cell.
Second, I did get chrome deposited but not great. The deposit is uneven, with areas of copper still showing through, and in places the deposit is
heavy, dark gray and dull (the copper cathode was thoroughly cleaned beforehand). OTOH the deposition is fairly quick, in less than 1 minute I got the
deposit as above and further electrolysis time (up to 10 minutes) didn't improve on it. Using a lower current of 30mA resulted in a similar deposit
forming in about 5 minutes, on which extra time (up to 30min) didn't improve.
Third, the chromium plating isn't great. It is reasonably adherent overall, but with weak spots where it can easily get dislodged by light polishing.
Since the appearance of the deposit off the bath was quite ugly I tried to improve on it by polishing with a chromium oxide paste. Now, Cr2O3 is a
pretty harsh abrasive, granted. But light rubbing of the paste over the plated area with only light pressure with the tips of my fingers removed large
areas of the deposit. What was left is pretty strong and adherent and was not easily removed with extended polishing; its appearance is also very good
and shiny after the extra polishing. But it only covers about 50% of the plated area, the remaining is gone.
No pics, I did not want to get chromium oxide onto my lens. 
Not a complete failure, but also not very encouraging.
(edit) I did try higher current densities up to 200mA/cm^2 but that required higher voltages and led to significant gas generation with a lot of
bubbling especially at the cathode, that's why I lowered it to 100mA/cm^2 and below; the plating obtained with the higher currents was also
significantly worse with lots of pitting.
[Edited on 27-1-2019 by stamasd]
|
|
|
stamasd
Hazard to Others
  
Posts: 133
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I forgot to mention in the post above that the liquid's impedance seemed to vary nonlinearly with the voltage. Actually I should say that the cell
impedance varies nonlinearly (as I used physical electrodes with it, not perfect electrodes). When varying the voltage from 0V to 24V, initially no
current passed up until 4V; at 5V the current was 0.01A; at 6V, 0.03A; at 9V, 0.1A; at 15V, 0.2A; at 24V, 0.3A.
Attaching a little quick-and-dirty chart.
Almost looks like the current profile for a diode.
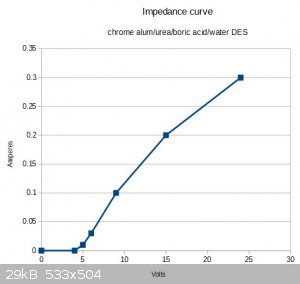
[Edited on 28-1-2019 by stamasd]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4298
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That makes sense as far as I understand it- there won't be any conductivity unless there's a redox reaction at both electrodes, and that won't happen
unless the voltage applied is enough to force the reaction to go.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
|
Thread Pruned
1-2-2019 at 16:49 |
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
If you are into ester chemistry, aroma compounds, the DES formed from choline chloride and glycerol can extract fatty alcohols from impure esters.
E.g. it can extract 1-nonanol from nonyl acetate, a mixture which would result from fischer esterification which doesn't go to completion.
https://www.sciencedirect.com/science/article/pii/S004040391...
I am trying this out right now, i just prepared 80 mL of DES from 1:2 molar equivalent of ChCl and glycerol. I have samples of nonyl acetate that are
contaminated with the smell of 1-nonanol. Will post a lab report after I try purifying them, but I can't analyze purity b/c no access to university
instruments during COVID-19, only report the smell (nonyl acetate is mushroom, 1-nonanol is citrus)
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2722
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
While searching for something else, I discovered that dimethyl urea and tartaric acid form a salt-free organic DES:
https://www.tandfonline.com/doi/full/10.1080/00397911.2021.1...
Unfortunately I am not sure if it should be symmetric or asymmetric dimethyl urea — the former seems a little easier to make, if the rxn of
methylamine with dimethyl carbonate works.
|
|
|
| Pages:
1
2
3
4
5 |