| Pages:
1
..
8
9
10
11
12
..
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ok, Mr Action 'the-others-just-keep-on-thumb-fiddling' Man, it sounds like a plan. Approved [insert sound of rubber stamp]!
Quote: Originally posted by aga  | OK.
In the reaction scheme, the acetone is absent.
If it is merely a solvent, then the sheer quantity specified is vastly greater than it needs to be : during reaction the drip rate from the condenser
is around 2 drops a second or less @ 85 C.
[Edited on 27-12-2015 by aga] |
The acetone is there to make the catalysis homogeneous, which makes things faster. For now, let's not touch the reagent/reagent
product ratios YET.
Will now watch U2U video.
Thank you, please! 
[Edited on 27-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It does seem to my barely trained eye that this synthesis has not been thoroughly investigated ... Yet !
That makes it More interesting !
Ratios remain in place ... for now.
The Dark Side Beckons ...
Reminds me : where is Darkstar when needed ?
Surfing atmospheres and calling himself 'Talby' most likely.
https://en.wikipedia.org/wiki/Dark_Star_%28film%29
[Edited on 28-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ratios may affect ratios of isomers: alpha, beta, gamma terpineol. Just a thought. Definitely worth investigating though.  
[Edited on 28-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A quick word about heterogeneous and homogeneous reactions/catalysis.
The catalyst here is dilute aqueous H2SO4, in which α-pinene is insoluble (more or less). When sufficient acetone is added we
get a three component one-phase system: homogeneous.
The latter is broadly preferred because of better contact between the reagents, which equals higher number of collisions.
Heterogeneous requires mass transfer between the phases to get collisions between reagent(s) and catalyst.
I believe that's the rationale behind using a weakly acidic acetone solvent here but that certainly doesn't mean trying a heterogeneous approach isn't
worth attempting, at some point.
[Edited on 28-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Bloggers returns to the lab (slowly!)
After my Annus Horribilis (2015) I'm ready to return to my lab. After a little trouble getting into it (frozen lock!) and getting the lights
to work (damp!) today I'm conducting a simple experiment.
Some time ago I bought some terpineol here. This is the naturally occurring isomer mix and NOT pure alpha-terpineol. This product had previously been tested by me as catalyst for the KOH/Mg reduction,
with negative results.
It's a mildly viscous liquid, perfectly clear and with a faint, pleasant floral odour, distinctly different from turpentine (which I also have).
I've simply poured a few ml into a clean test tube, covered it and put this into an ice bath. Will anything crystallise out?
Wait and see...
[Edited on 29-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Get a distillation going to warm the lab up.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It's got heating but it cut out when left too long unattended. Quite a bit of clean-up to do as well.
Ah, life!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
In 5 minutes the next attempt will have finished refluxing.
This is 20ml alpha-pinene in 40ml 15 v/v% acid and 250ml acetone.
I'll split off 125ml of the reaction liquid and chuck K2CO3 into it until it stops fizzing and see what happens.
The rest will be put aside to be subjected to whatever process you desire (and i have the ability to do).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Awaitng progress report in Real Time. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Strange Results
Post reflux the liquid was once again a clear colourless liquid.
125ml was spilt off from the reaction mixture of 310ml.
This portion was cooled rapidly in a water bath and the liquid became cloudy same as last time.
After a few minutes standing, the cooled portion started to clear and a colourless layer was seen on top of the cloudy liquid (N.B. this is with NO
NaOH added).
After another 5 minutes the cloudy lower layer began to clear from the bottom up.

The liquid was swirled to re-mix the layers and two portions of 3ml were drawn off with a pipette and placed into two test tubes.
A spatula tip of K2CO3 was added to one and 1ml of 15w% NaOH was added to other.
Both were shaken and allowed to stand for 5 minutes.
The K2CO3 portion formed a thin upper layer of water-clear liquid and a yellow/brown cloudy lower layer.
The NaOH portion formed 3 layers !
The upper layer was water clear, the middle layer cloudy, and the lower layer appeared as globules of a clear oil-like substance.

Carefully removing the lower layer with a pipette, a drop was put on some absorbent kitchen towel to test the smell (everything smells of acetone).
Immediately on touching the towel, clear crystals formed.
The rest of the sample was added to the towel to create a larger batch of crystals.

[Edited on 30-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
What happened to the rest of the 310 ml of post-reflux mixture? That contains about half of the alpha-terpineol!
At this point my guess is that the crystals are Na2SO4. The lower layer MUST be aqueous. Density dictates that. The upper layer
MUST be the organics.
Please carefully check the water solubility of the crystals. Na2SO4 is highly water soluble, alpha-terpineol is not.
************
My experiment yielded nothing. No crystals after 4 h at 0 C. No crystals overnight at -18 C.
[Edited on 30-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's in a stoppered RBF awaiting instructions.
Quote: Originally posted by blogfast25  | | At this point my guess is that the crystals are Na2SO4. The lower layer MUST be aqueous. Density dictates that. The upper layer
MUST be the organics. |
Matters not. I cannot repeat that result, and it's not what we're after anyway.
Same here with what i just tried on 50ml of the 125ml portion.
The clear upper layer was separated and then NaOH solution added to the remainder in a sep funnel, capped, shaken, vented etc.
Separation happened.
Boiling off most of the acetone caused the remainder to become a yellowish liquid with an oily film on top.
Funnily enough, the pre-NaOH clear upper liquid also reacts with NaOH forming 2 phases.
Pretty sure i'm lost now, partly due to acetone intoxication.
Time for some emergency Medication 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nice work.
I is a tizzy cornfused now. By rights we should have 10 to 20 ml of organic phase now, presumed mainly alpha-terpineol.
Amirong? Or isiright?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
We got 185ml of post-reflux mixture which has a clear upper layer already, without adding any NaOH.
There is also a watch glass with some yellow stuff on it and a load of glassware to clean.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | We got 185ml of post-reflux mixture which has a clear upper layer already, without adding any NaOH.
|
I suggest to neutralise, allow to stand, the separate organics.
Perhaps mildly chill to see if we get crystals?
Then tell the mice to do the washing up.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
As we already have a separation of two phases, surely the upper phase should be removed before doing anything, as it must contain a different compound
to that in the lower phase, or is that too simplistic ?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
With the last 60 ml of 'my' 125ml portion i've removed the top layer (added it to the previous ones) then neutralised the acid with NaOH , separated
the upper layer and put it in a 250ml beaker so the acetone can evaporate off overnight.
Ve vill zee vot 'appens.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Blogfast, it sounds as though the mixed isomers in your terpineol are suppressing the melting point. Perhaps putting some more effort into purifying
the alpha pinene beforehand will help to simplify the reaction workup for aga. 35C is quite low for a MP, so I'm not really surprised that the
terpineol isn't solidifying.
The benzoate ester should be a solid despite melting point depression however, so it might be worth a try esterifying some of the suspected terpineol,
then recrystallizing it and saponifying it. Should be quite high yielding due to terpineol's low water solubility.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | As we already have a separation of two phases, surely the upper phase should be removed before doing anything, as it must contain a different compound
to that in the lower phase, or is that too simplistic ?
|
Yes. You can remove the OL w/o neutralising.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | Blogfast, it sounds as though the mixed isomers in your terpineol are suppressing the melting point. Perhaps putting some more effort into purifying
the alpha pinene beforehand will help to simplify the reaction workup for aga. 35C is quite low for a MP, so I'm not really surprised that the
terpineol isn't solidifying.
The benzoate ester should be a solid despite melting point depression however, so it might be worth a try esterifying some of the suspected terpineol,
then recrystallizing it and saponifying it. Should be quite high yielding due to terpineol's low water solubility. |
Agreed with both points. I didn't expect much of the freezing at all.
Got a recipe for esterification to benzoate? That must take a while, with all that steric hindrance...
And I'm a bit concerned about the double bond in there, with conc. H2SO4 as catalyst...
[Edited on 30-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gdflp  | | Perhaps putting some more effort into purifying the alpha pinene beforehand will help to simplify the reaction workup for aga. |
Suggestions?
All i've done so far is distill commercial turps.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by blogfast25  |
Agreed with both points. I didn't expect much of the freezing at all.
Got a recipe for esterification to benzoate? That must take a while, with all that steric hindrance...
And I'm a bit concerned about the double bond in there, with conc. H2SO4 as catalyst...
|
That's why I was thinking benzoyl chloride, especially since aga likes making disulfur dichloride. That would alleviate the issues due to steric hindrance, and with proper reaction conditions, HCl wouldn't be an
issue with the double bond. That would alleviate the issues due to steric hindrance, and with proper reaction conditions, HCl wouldn't be an
issue with the double bond.
@aga As for purifying the terpene, I can't think of anything right now. I didn't find anything in <i>Purification of Laboratory
Chemicals</i>. I'll update if I think of anything.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
S2Cl2 is a complete hooligan.
It does not have any manners at all and just barges through organic molecules like a randy Bulldozer chasing a Cowdozer on heat.
[Edited on 30-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Esterification of a t-alcohol (carbinol) with benzoyl chloride
(I think  ) )
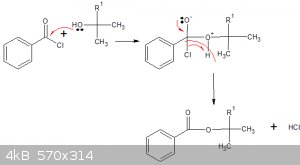
The question is, how selective would such an esterification be with regards to the 4 terpineol isomers? W/o selectivity, no basis for
separation.
[Edited on 30-12-2015 by blogfast25]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
It's not selective in the least. But, assuming that the crude is reasonably pure a-terpineol, it allows a convenient method of purification by
recrystallization from a suitable solvent such as methanol.
|
|
|
| Pages:
1
..
8
9
10
11
12
..
19 |