| Pages:
1
..
10
11
12
13
14
..
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Plan?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes, that would be a good idea.
Doesn't seem 100% likely though.
Boil stuff a lot will probably feature heavily.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
http://www.hekserij.nl/alfa_Terpineol.aspx
hekserij.nl is a small Dutch outfit I've bought stuff from before. Good service, no problems.
This grade is about 90 %, so would still need some refining. €2.5 for 10 ml (shipping and tax not incl.) is not the worst price ever.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
No postage to Spain sadly.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I would buy it and send it to Viva Espana, por favor!
One thing that might still worth trying is to replicate your very first attempt, the one that yielded some crystals...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It would be good to see some actual Product if only for a reference.
Quote: Originally posted by blogfast25  |
One thing that might still worth trying is to replicate your very first attempt, the one that yielded some crystals...
|
That is a better plan.
Just 'giving up' is not an option.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ok, it's a (2 point) deal, hombre! 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Was doing the reflux thing some more today.
Doesn't have to be all done in one day, so may as well do an hour or so more refluxing each day for a week.
I noticed that the mix with acetone isn't actually homogenous. The demarcation is very subtle, but there are definitely two phases, so it's not as
ideal as first thought.
That write-up is starting to sound either bogus, or useless for actual production, seeing as you need a GC to separate the micrograms of product.
The acetone bothers me a lot: boiling at 56 C means the reaction mixture is colder than it could be without the acetone.
I worked out the enthalpy to be around <strike>+313 kJ/mol</strike> (see below) which would suggest it needs hotter conditions to proceed
at any worthwhile rate.
Suggestion: pre-mix the alpha-pinene and 36% acid (plenty of water already included) then bang it in a pressure cooker for a few hours.
The pressure will force the whole thing to higher temps and there should be a whole load of superheated vapours available for the hydrogenation
reaction with the pre-protonated alpha-pinene.
Whaddyall think ?
[Edited on 4-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Was doing the reflux thing some more today.
I. Doesn't have to be all done in one day, so may as well do an hour or so more refluxing each day for a week.
II. I noticed that the mix with acetone isn't actually homogenous. The demarcation is very subtle, but there are definitely two
phases, so it's not as ideal as first thought.
III. That write-up is starting to sound either bogus, or useless for actual production, seeing as you need a GC to separate the
micrograms of product.
IV. The acetone bothers me a lot: boiling at 56 C means the reaction mixture is colder than it could be without the acetone.
I worked out the enthalpy to be around +313 kJ/mol which would suggest it needs hotter conditions to proceed at any worthwhile rate.
V. Suggestion: pre-mix the alpha-pinene and 36% acid (plenty of water already included) then bang it in a pressure cooker for a few
hours.
The pressure will force the whole thing to higher temps and there should be a whole load of superheated vapours available for the hydrogenation
reaction with the pre-protonated alpha-pinene.
Whaddyall think ? |
I. True but splitting it up does reduce control over time. See (from that paper):
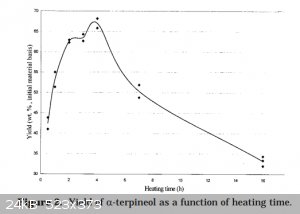
II. That's interesting but acetone is still likely to act as a mass transfer agent.
III. I'm not giving up on it yet. In principle there's no reasone why it can't be scaled up.
IV. My value of EoR is also positive but a bit lower than yours. Either way, heat pushes endothermic equilibria to the right. The
main effect of temperature is on reaction rate though.
V. Pressure cookers have two main disadvantages here:
1. Corrosion: hot dilute H2SO4 is the enemy of aluminium or SS.
2. Temperature is max. about 121 C, usually 118 C. Not as high as you might think. But even 20 C can make a lot of difference in terms of reaction
rate.
[Edited on 4-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Using the table from the QM/OC thread :-
http://www.sciencemadness.org/talk/viewthread.php?tid=62973&...
... according to the reaction mechanism here :-
http://www.sciencemadness.org/talk/viewthread.php?tid=15171&...
we break bonds :-
step 1: C-C 368
step 2: C-C 368
step 3: O-H 465
total broken: 1201
we make bonds :-
step 1: C-H 414
step 2: C-C 368
step 3: C-O 352
total made: 1134
Which gives 67kJ/mol required, not 313.
Sorry.
I was sober at the time and obviously not thinking clearly.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
+67 kJ/mol is almost 'neutral'. Temperature won't affect equilibrium that much. And we're supplying heat anyroads.
[Edited on 4-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Really ? (i still have not got a handle on what the kJ/mol thing feels like).
In that case we're facing Kinetic barriers to a sucessful reaction.
Hmm.
[Edited on 4-1-2016 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Idea: any clue as to how much H2O converts to H3O<sup>+</sup> at a given temperature ?
Perhaps there simply is not much actual H2O available in the water.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I. Really ? (i still have not got a handle on what the kJ/mol thing feels like).
II. In that case we're facing Kinetic barriers to a sucessful reaction.
III. Hmm.
|
I. Classic Thermite is about - 850 kJ/mol (of Fe2O3), e.g.
II. I think we made some alpha-T, that first run. It's driven by entropy, not enthalpy, this one.
III. Erm.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Best write a description of how Entropy relates to alchemy, probably best in the QM/OC thread.
I'm out of my depth here and paddling in acetone, which is not ideal for an alcoholic chain smoker.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I'm out of my depth here and paddling in acetone, which is not ideal for an alcoholic chain smoker. |
Do you run these refluxions in the fume hood?
As regards Entropy determination:
Quote: Originally posted by aga  | Best write a description of how Entropy relates to alchemy, probably best in the QM/OC thread.
|
...bar for the simplest of molecules Entropy can only be determined experimentally, see e.g. here.
$$\Delta S=\frac{Q_{rev}}{T}$$
Yeehaw!
Here is an example of the empirical determination of the Entropy of Al2O3:
http://www2.stetson.edu/~wgrubbs/datadriven/entropyaluminumo...
[Edited on 5-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A change of tactic?
alpha-terpineol from turpentine patent:
http://www.google.co.uk/patents/US2898380
See "Example 1."
Filed by American Cyanamid Co, quite a reputable company.
There is actually one quite interesting factoid to be gleaned from it. Will elaborate tomorrow.
[Edited on 7-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|



These are taken from the patent source text linked to above.
The authors assert that residual H2SO4 is difficult to wash out of the crude hydrate and that this is a cause of low
alpha-terpineol yield.
Something to consider?
[Edited on 7-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Interesting patent info - great find bloggers.
No acetone i see. Good !
Still cannot understand the huge wash of acetone in the previous experiment (which didn't work).
Got no IGEPAL CO-630 (octylphenoxypolyethoxyethanol) non-ionic emulsifying agent handy so did a couple of little experiments with washing up liquid
instead.
Works fine - the mixture of that with acid & pinene goes milky white with mild stirring.
The acid rips out the green colour, so it is no longer a Mild Green Stirry liquid<sup>1</sup> 
Too little WUL and the layers separate quickly.
Too much and it'll probably go all frothy.
So now there's a pot with 53g of turps, 189g 36% H2SO4 and 1.15g of washing up liquid happily stirring in a 250ml FBF at around 23 C (ambient)
If the claimed yield can be achieved, that's 33ml turpineol.
Doubtless it will end up a lot less due to idiocy, and the starting material has not been distilled this time.
<font size="1"><sup>1</sup> Close to a very old UK advert slogan for a famous washing up liquid brand, for you non-UK
people.</font>
[Edited on 7-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
Nice work!
I suggest for work-up the procedure of 'Sample B', then distil at atm. pressure, closely monitoring head temperature. Probably leave the fraction of
BP > 170 - 180 C in the pot.
Then all will be revealed.
Ultima Ratio Regum!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Vac pump can pull 66 mBar, just not so happy about imploding glassware.
cum fortuna braccae non dissiliunt
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You do realise that even at 0 mm Hg, the pressure acting on the glass is still only 1 bar, right? That's like taking an RBF, stoppering it and taking
it on a scuba dive of 20.66 m deep. You think it would implode?
As far as I understand glassware for vac distillation requires no special specs.
But here atm. dist should still work, just don't try and get the alpha-terpineol to come over!
And if everything comes over at abt. 155 C we know it's another epic fail. 
Parolum me posterium exit?
[Edited on 7-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yeah. OK. The key is by the teapot.
Chinese glass, and not tried it before under vacuum, so standard distillation conditions preferred for now.
Scuba at 50m in Barbados was interesting seeing as my assigned 'dive buddy' immediately proclaimed his asthma and epilepsy.
Worse was water skiing - the fastest way to an instant saltwater enema i have ever discovered.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I was looking for it in the pot marked 'Madras Paste'. Sorry.
Incidentally, I'm not sure what this 'Sample B' post-reaction addition of fresh alpha-pinene is supposed to actually do but it's worth a
shot, IMHO.
[Edited on 8-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Another batch of alpha-terpineol has been prepared as per Example 2 of that last patent bloggers found, then separated, then washed and refluxed with
more acid, then distilled.
5.42g of a yellowish liquid is what remains when the head temperature went over 170 C.
The Big question is : how can i tell what it is ?
Edit :
Two more batches also under way.
[Edited on 9-1-2016 by aga]
|
|
|
| Pages:
1
..
10
11
12
13
14
..
19 |