| Pages:
1
..
11
12
13
14
15
..
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Another batch of alpha-terpineol has been prepared as per Example 2 of that last patent bloggers found, then separated, then washed and refluxed with
more acid, then distilled.
5.42g of a yellowish liquid is what remains when the head temperature went over 170 C.
The Big question is : how can i tell what it is ?
Edit :
Two more batches also under way.
[Edited on 9-1-2016 by aga] |
| Quote: | | So now there's a pot with 53g of turps, 189g 36% H2SO4 and 1.15g of washing up liquid happily stirring in a 250ml FBF at around 23 C (ambient)
|
5.42 g of product from 53 g of turps? Not exactly promising... How long did you agitate for? At < 10 % yield, this would not be worth pursuing.
Again, try and chill to see if anything crystallises?
[Edited on 9-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Initially the mixture is spun up for 20 to 24 hours.
The "Turpentine Oil Hydration using Trichloroacetic Acid as Catalyst" paper abbreviates the catalyst to TCA, so i thought, why not try
trichlorocyanuric acid (TCCA) ?
Done.
No bubbles of lethal gas evolved, which was a bit of a concern.
Looks more promising, as the upper layer is at least smaller than with the previous attempts, and separates to water-clarity in the reaction vessel.
It's been separated and is cooling as i type.
Maybe crystals in the morning.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Initially the mixture is spun up for 20 to 24 hours.
The "Turpentine Oil Hydration using Trichloroacetic Acid as Catalyst" paper abbreviates the catalyst to TCA, so i thought, why not try
trichlorocyanuric acid (TCCA) ?
Done.
|
Trichloroacetic Acid is used there because it's a very strong acid.
Trichloroisocyanuric acid is a very weak acid. This won't work. You might chlorinate that double bond in alpha-pinene somewhat but that's
all...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Bugger.
Edit:
Wait a sec.
The other paper claims that the presence of a strong acid reduces yields.
Guess we'll see if there's crystals in the morning.
[Edited on 9-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Got a link to the paper with trichloroacetic acid as catalyst?
Without a minimum of H3O<sup>+</sup> the hydration cannot proceed because of that first step: electrophylic attack on that
double bond:
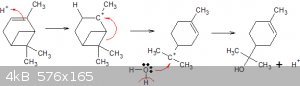
... to create that 'wandering' carbonium ion.
Super strong acids are not needed here because we're in the presence of water anyway.
We might have to start thinking about going back to the original plan:
alpha-pinene + glacial acetic acid (GAA) === > alpha-terpineol acetate. Then de-esterify the acetate to get the precious carbinol!
Got any GAA?
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Can't be bothered trimming the link. Here's the pdf.
Attachment: Ajoct.Vol-1.-Issue-9.-21-26(1).pdf (206kB)
This file has been downloaded 650 times
Likely that the acid catlysis mechanism is far more complex, and the carbonium do-dah is just a part of it.
Edit:
GAA is on hand, maybe 150ml.
More can be made if needed (have a big pot of sodium acetate and lots of conc sulph).
[Edited on 10-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks for the *.pdf. TCA is hard to get and expensive.
Quote: Originally posted by aga  |
I. Likely that the acid catlysis mechanism is far more complex, and the carbonium do-dah is just a part of it.
II. GAA is on hand, maybe 150ml.
|
I. The mechanism is sound, trust me: it's highly explanatory. But side-reactions do also take place. It's the same mechanism for
alpha-terpineol acetate with GAA, BTW...
II. Hold fire with that. ('No stupid moves and no one get hurt, mister!')
Let's formulate a plan ('failing to plan equals planning to fail').
The advantage of GAA is that we can measure it: by... titration! YOUR FAVOURITE method! Talk about 'clouds and silver linings!!!'  
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
TCCA gave nothing apart from a slight chlorine smell.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Trichloroacetic Acid is used there because it's a very strong acid.
Trichloroisocyanuric acid is a very weak acid. This won't work. You might chlorinate that double bond in alpha-pinene somewhat but that's
all... |
Trichloroacetic acid with its pKa of 0.66 is hardly a "very strong acid". It is strong, but still a few million times weaker than HCl (in water).
Trichloroisocyanuric acid is not an acid at all (except by name). That is unless you want to call any electrophile as an acid, which would however
make the phrase acid obsolete. (Though sometimes they are called acid if that is their main role in the reaction. For example, I2 is often
called an acid when it is used as a soft acid catalyst.)
The outcome of its reaction with alpha-pinene depends on the solvent used, but in most cases it should give a mixture of products due to the
Wagner–Meerwein rearrangements of the reaction intermediates.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The data from the 2013 paper in the pdf above states that 88% of the alpha-pinene is converted in 30 minutes @ 70 C with 87% selectivity for
alpha-terpineol, when trichloracetic acid is used as the catalyst, with water and acetone as the solvent(s).
Page 99 of "Practical Organic Chemistry, J B Cohen" (available in the SM library)
details the production of chloral hydrate and trichloracetic acid, and it appears that all of the reagents are available.
Would it not be a better plan to synthesise some TCA and follow the 2013 route to alpha-terpineol ?
It seems that chloral hydrate is not a good thing to have lying around.
If it is quickly converted to TCA, can anyone see a downside ?
Edit:
How much terpine do we want at the end of this ?
Working out the figures, to get 8ml of product the process requires 106g of TCA, if the ratios must be held to.
This in turn means making 266g minimum of chloral hydrate.
[Edited on 10-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very good research, aga.
Let's keep TCA on hold for a minute but it could be useful also with regards to my next post on limonene as a precursor, instead of alpha-pinene.
We need about 10 ml of alpha-terpineol. Bear in mind it's not our end-product!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And then there's limonene...
Limonene is very OTC and very cheap and a known precursor to α-terpineol or its acetate:
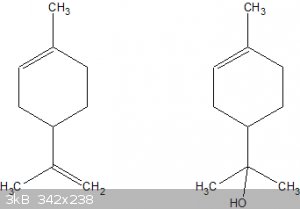
(Left: limonene, right: α-terpineol)
The bottom double bond can be hydrated using basically the same methods as used for α-pinene to α-terpineol or its acetate by means of
H2SO4, TCA, GAA (to the acetate) etc.
An excellent monography on limonene's chemistry can be found here.
In short, there's nothing that can be achieved with α-pinene that can't be achieved with limonene but generally speaking better: more
selectively, with fewer by-products and even more selectively (I believe) when the acetate is the target.
One reaction of interest is the electrophylic addition of HCl to limonene in anhydrous conditons (using DCM as solvent), which reportedly yields
α-terpineol chloride:
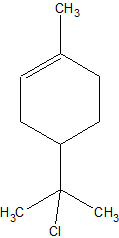
Which can easily be hydrolysed with NaOH(aq) to α-terpineol.
So, all in all, we still have a few possibilities open to us.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
Trichloroacetic acid with its pKa of 0.66 is hardly a "very strong acid". It is strong, but still a few million times weaker than HCl (in water).
|
Oooopsie. Obviously I hadn't looked up the Ka. Thank you for the correction.
[Edited on 10-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Page 99 of "Practical Organic Chemistry, J B Cohen" (available in the SM library) details the production of chloral hydrate and trichloracetic acid,
and it appears that all of the reagents are available. |
Which gives me:
"Chloral Hydrate, CC13.C Liebig, A?inale7i, 1832, 1, 189; Dumas, Aim. CJiim. Phys. 1834, 56, 123. Chloral hydrate is obtained by the action of
chlorine upon ethyl alcohol The solid chloral alcoholate is formed, CC13.CHOH.OC.2H5, which, when decomposed with sulphuric acid, yields chloral,
CC13.COH, a liquid which combines with water to form the crystalline hydrate. Properties.—It crystallises in prisms, which dissolve easily in water,
alcohol, and liquid hydrocarbons. It has a peculiar smell ; m. p. 57°; b. p. 97*5°. It volatilises on evaporating its aqueous solution.
Reactions.—1. Add a few drops of a solution of chloral hydrate to a little ammonio-silver nitrate solution and warm. Metallic silver will be
deposited. 2. Add a little caustic soda to a solution of chloral and warm gently. The heat of the hand is sufficient for the purpose. A smell of
chloroform is at once apparent, CC13.CH(OH)2 + NaOH = CHCl3 + HCO.ONa + H.>O. Sodium formate remains in solution. 3. Add a few drops of ammonium
sulphide solution and warm gently. A brown colouration or precipitate is formed.
Trichloracetic Acid, CC13.CO.OH. Dumas, Compt. rend., 1838, 8, 609 ; Clcrmont, A?in. Chim* Phvs., 1871, (6), 6, 135- 25 grins, chloral hydrate 20 „
fuming nitric acid ; sp. gr. I '5 (see p. 20)."
I couldn't see the end of the TCA synth but 'fuming nitric acid', do you have that?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Have made WFNA a couple of times.
Well, i think it was WFNA - it was colourless and fumed when blown on.
Using the last of the 99%+ ethanol (~150ml) in a test of the chlorination step it appears that the yield of solid 'chloral alcoholate' is also quite
small.
Smells almost exactly like wart remover.
Best get some more ethanol distilled.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Using the last of the 99%+ ethanol (~150ml) in a test of the chlorination step it appears that the yield of solid 'chloral alcoholate' is also quite
small.
Smells almost exactly like wart remover.
Best get some more ethanol distilled. |
So you got some solids? Perhaps more chlorine was needed?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
An example of (hydro)chlorination, without solvent and with dry HCl:
http://www.orgsyn.org/demo.aspx?prep=CV1P0166
I wonder if this would work for limonene?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes. There's a white solid in the filter paper at the moment, drying.
It looks like as much was made as is practicable.
What happens is the the white solid starts to form, then all of the ethanol goes milky, then the solids drop out, leaving a cloudy (yet transparent)
layer above.
No matter how much more chlorine was introduced, the transparent layer would not change, and the solid layer would not increase in depth, so it was
presumed 'done'.
Wiki says it's soluble in ethanol and water, so the remaining liquid is being left to evaporate away, which might yield more solids.
The fumes certainly impart a slight drunken sensation - trust me, i'm an Expert !
Certainly needs to be done in the fume hood, by someone expendable.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
Nice!
Could you cut and paste the conversion of the chloral hydrate to TCA for me here? That part of the *.pdf doesn't play for me.
The TCA might also be part recoverable after the hydration.
Someone expendable: get a miniaga!
I'm still not convinced of the rationale behind using TCA instead of e.g. H2SO4. Strong acids completely deprotonate in water:
HA(aq) + H2O(l) === > H3O<sup>+</sup>(aq) + A<sup>-</sup>(aq)
This means the protonating agent (Lewis acid) is H3O<sup>+</sup>(aq), not HA.
And that means that two strong acids, say HA1 and HA2, are used at the same aqueous concentration they should have
roughly the same catalytic effect...
It would make a difference in anhydrous conditions.
'These things are sent to try us'
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Chloral hydrate is obtained by the action of chlorine upon
ethyl alcohol. The solid chloral alcoholate is formed,
C13.CHOH.OC.2H5, which, when decomposed with sulphuric
acid, yields chloral, CC13.COH, a liquid which combines
with water to form the crystalline hydrate.
...
Trichloracetic Acid, CC13.CO.OH.
...
25 grms, chloral hydrate
20 „ fuming nitric acid ; sp. gr. I '5 (see p. 20).
The chloral hydrate is melted in a distilling flask (250 c.c.) and
the fuming nitric acid added.* The mixture is heated carefully
over a small flame until the reaction sets in. After a few
minutes red fumes are evolved, consisting mainly of nitrogen
tetroxide. The reaction proceeds without the application of
heat, and is complete when, on warming the liquid, nitrous
fumes cease to come off. The product is now distilled ; below
123° excess of nitric acid distils; between 12f and 1940 a.
mixture of trichloracetic acid and a small quantity of nitric acid
pass over, and at 194—1960 nearly pure trichloracetic acid
collects in the receiver and solidifies on cooling. It is advisable
to distil the last fraction with a condenser-tube only. The
fraction boiling at 123—1900 is treated with a fresh quantity of
fuming nitric acid (10 c.c), and the product purified as before.
Yield, 10—15grams.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  |
I'm still not convinced of the rationale behind using TCA instead of e.g. H2SO4. Strong acids completely deprotonate in water
|
No idea.
As i said, the mechanism could well be hideously complicated with plenty of intermediates, which would explain why so much acetone is specified, and
why Nicodem says :-
Quote: Originally posted by Nicodem  | | The outcome of its reaction with alpha-pinene depends on the solvent used, but in most cases it should give a mixture of products due to the
Wagner–Meerwein rearrangements of the reaction intermediates. |
Guess that somebody who can understand them takes a look at the Wagner–Meerwein rearrangment(s). I took a peek and can follow the blue line, but no
idea why the electrons move as they do.
All i know is that the 3.5 hour and 24 hour processes with sulphuric as catalyst gave next to nothing.
If that 2013 paper is right, there's approx 77% yield (based on pinene) after 30 minutes when TCA is used.
[Edited on 10-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Apart from Wagner–Meerwein rearrangment(s), other common by products like β and γ-terpineol can easily be explained:
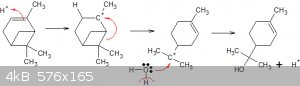
If the water attacks the carbonium cation of the type second in the series then we get the beta and/or gamma type isomers. Thus it's a question of
creating conditions in which that structure transits to the third kind as quickly as possible, in order to maximise alpha yield.
QC/OC thread to re-start soon, this time on carbanion ions: CH3<sup>-</sup> and such like...
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The catalyst (the acid) is clearly explained in your mechanism, however it can at best be a Summary of the reaction mechanism.
How is the Solvent-dependant product mix explained if the solvent has no part in the reaction ?
Similarly, how can it be that TCA had the highest selectivity for alpha-terpineol compared other tested catalysts if were merely supplying protons ?
The experimental data strongly suggests that Other steps are happening, not just the formation of a wandering carbocation.
Not that i know jack shit really, just seems logical.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
In this JPL paper
https://www.researchgate.net/publication/231665911_Heterogen...
they say :-
"Acetone was found to be physically absorbed by sulfuric acid without undergoing irreversible reaction below acid concentrations of 87 wt. %"
Above 87 wt % they saw 'condensation products' such as mesityl oxide.
This suggests that the 'simple' mix of acetone and our sulphuric acid catalyst is far from simple.
Why would the acetone react above 87% and not react below 87% ?
Makes no sense unless that system is complex, with the components reacting in an equilibrium, favouring acetone until there are so many protons (or
SO4<sup>2-</sup> around that another species is favoured around that another species is favoured
[Edited on 10-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I. The catalyst (the acid) is clearly explained in your mechanism, however it can at best be a Summary of the reaction mechanism.
II. How is the Solvent-dependant product mix explained if the solvent has no part in the reaction ?
III. Similarly, how can it be that TCA had the highest selectivity for alpha-terpineol compared other tested catalysts if were merely
supplying protons ?
IV. The experimental data strongly suggests that Other steps are happening, not just the formation of a wandering carbocation.
|
I. It also explains why we end up with an alcohol (or an acetate in the case of GAA). What isn't explained is why the
carbocation moves. That has to do with the third one being of lowest energy. But I don't know why...
II. Already explained: any OC will tell you that where possible homogeneous catalysis is preferred. That's the role of the
acetone: make it all one phase.
III. That is merely a claim by one author. Have you seen his back-to-back comparisons with other catalysts anywhere?
He may be right about selectivity but he certainly has not proved it. Has he shown a meta-analysis somewhere?
IV. No dispute. It's already been shown which kind rearrangements and by-products can be expected/explained. Careful not to resort to
'it's magic!' kind of explanations!
[Edited on 10-1-2016 by blogfast25]
|
|
|
| Pages:
1
..
11
12
13
14
15
..
19 |