| Pages:
1
..
9
10
11
12
13
..
19 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Think i'll go and have a word with a pine tree in the morning for alternate raw material.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | Think i'll go and have a word with a pine tree in the morning for alternate raw material. |
Why? Please remember that turpentine is in fact distilled pine resins, whatever is understood by distillation here.
https://en.wikipedia.org/wiki/Turpentine#Converting_oleoresi...
So far the (re-distilled) turpentine has served quite well, I think.
[Edited on 31-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@gdflp:
What I might try is to treat my commercial isomeric terpineol mixture with 20 % HCl at RT. t-alcohols halogenate easily. Then dry and freeze again.
Perhaps some of these R-Cl might then drop out...
[Edited on 31-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
There's a big pine tree a few hundred metres away.
I like trees, and will enjoy going to have a look at it.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Indeed, since the reaction will happen at RT, you might be able to get away with forming the alkyl chloride with minimal hydration of the double bond.
It might provide some separation from certain impurities, but I don't see why the other isomers of terpineol wouldn't chlorinate as well, and I don't
see how the alkyl chloride will be any easier to purify than the alcohol.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | | Indeed, since the reaction will happen at RT, you might be able to get away with forming the alkyl chloride with minimal hydration of the double bond.
It might provide some separation from certain impurities, but I don't see why the other isomers of terpineol wouldn't chlorinate as well, and I don't
see how the alkyl chloride will be any easier to purify than the alcohol. |
No contest. 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Stroke it for me. 
Biochemistry is amazing.
Below are tree, I mean three, structures that are almost certainly genetically related:
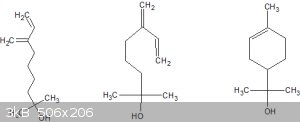
Left and middle are the same (rearranged) but note the similarity between middle and right. Middle is Myrcenol, a substance found in various pine-related plants. It's saturated version, tetrahydro myrcenol, is a target molecule of this project.
@gdflp:
I hope you're listening to all this because you're in charge of finding a way to convert right to middle!  
[Edited on 31-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
There are some crystals this morning.
Not all of the acetone has evaporated off yet, so i suspect there'll be more.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | There are some crystals this morning.
Not all of the acetone has evaporated off yet, so i suspect there'll be more. |
Good work so far, aga.
Allow time to do its work.
I propose to collect the crystals on a Buchner (with mild vacuum) and to wash them once with cold 50/50 v/v acetone/water. Then recover them from the
filter and air dry them for a few days.
If that plan doesn't flounder I'm tempted to move to Stage 2: hydrogenation, without further purification of the alpha-terpineol because we'll have to
work-up the saturated version anyway.
[Edited on 31-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Bad news : the crystals have buggered off.
Do they decompose in sunlight ?
Good news is that the acetone smell has gone.
I've put the beaker in a fridge to see if the crystals reappear.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Bad news : the crystals have buggered off.
Do they decompose in sunlight ?
Good news is that the acetone smell has gone.
I've put the beaker in a fridge to see if the crystals reappear. |
How many ml approx. do you have? Let's see what chilling does.
[Edited on 31-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
About 25 ~ 35 ml.
This is still the remains of the 125ml portion i used for messing with.
The larger volume of post-reflux liquid remains untouched.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Today the sample still shows no crystals, despite being in a fridge at 7 C overnight.
I've gone ahead and neutralised the acid in the remaining post-reflux 185ml liquid after separating the clear Upper layer, adding it to the collection
of post-reflux upper layers (which is also in the fridge).
After adding the NaOH two layers formed, so they were separated and the Upper layer is also in the fridge now.
The lower aqueous layer readily precipitates crystals of presumably Na2SO4, especially in the sep funnel !
Remember that we already have a phase sep after reflux, before neutralising the acid, and this collected Upper layer is sitting quietly in a beaker.
So, what next with the Two separated phases (pre-and post acid neutralisation) ?
[Edited on 1-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A teensie bit disappointing, this crystals-no-show.
I suggest to pool all organic phases together and wash them about three times with clean water, using a sep. funnel. This would remove any remaining
acetone and any other water soluble impurities.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sorry. No can do.
Mixing them up seems viscerally wrong.
The Upper phase that separated after the reflux Must be different to the stuff that separated after neutralising the acid.
This substance also shows phase sep on treatment with NaOH.
There are at least two products.
Maybe my thinking is wrong, in which case i'l do it all again again.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
The Upper phase that separated after the reflux Must be different to the stuff that separated after neutralising the acid.
|
Explain your reasoning? The neutralisation only serves to kill the catalyst, not to modify the product.
| Quote: | | Maybe my thinking is wrong, in which case i'l do it all again again. |
I see no reason to start all over again: all these organic phases must contain alpha-terpineol. It's a matter of getting it out of there.
If we don't get any crystals I think pooling together, washing with water, drying (over CaCl2 or MgSO4) and taking a BP should
be the next step.
Then perhaps trying to drive off some low-boilers. Unreacted alpha-pinene and stuff like 3-carene boil around 150 - 160 C, so distilling these off is
a real possibility...
Alternative plans are will be heard, of course. Think about it.   There's no fire and no rush. There's no fire and no rush.
[Edited on 2-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Distilling the upper layer from the post-reflux, pre-NaOH liquid, it came over entirely at around 160 C, so i guess it's unreacted pinene.
Distilling the post-reflux post-NaOH addition upper layer stabilised at 56 C for a while, paused briefly at 3 other temperatures before stabilising at
100 C.
The liquid left in the boiling pot did not form any layers, so it was cooled (nothing happened) then transferred to a sep funnel and the last few ml
(maybe 15) of the available DCM was added.
Shake, vent, wait, and two layers formed.
The Lower layer appears to be the product, which forms solids.
Currently it is in the fridge and reeks of DCM.
[Edited on 2-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I. Distilling the upper layer from the post-reflux, pre-NaOH liquid, it came over entirely at around 160 C, so i guess it's unreacted
pinene.
II. Distilling the post-reflux post-NaOH addition upper layer stabilised at 56 C for a while, paused briefly at 3 other temperatures
before stabilising at 100 C.
The liquid left in the boiling pot did not form any layers, so it was cooled (nothing happened) then transferred to a sep funnel and the last few ml
(maybe 15) of the available DCM was added.
Shake, vent, wait, and two layers formed.
The Lower layer appears to be the product, which forms solids.
Currently it is in the fridge and reeks of DCM.
|
I. Total mystery. Clueless in Bridlington. It might suggest to cook, neutralise and further process all the same day? Still doesn't
make a lot of sense...
II. Good move with the DCM! With density of 1.33 that must surely be the lower phase. Sulphate must be in top layer.
Try and quantify the amount of solids?
********
All that variability is worrying though. Good work nonetheless. I think we're nano-inching in the right direction! 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's a journey.
As far as i can tell, quite a few OC 'Literature' accounts are pure Lies or skip mentioning many many things.
This one Will be hammered Flat.
To Know is to Know.
To Lie about Knowing is pollution of the Knowlege for All.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Sapere Aude!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Eeek ! Polymer !
In the fridge the 'product' was a soild and stuck to the beaker, so some absolute ethanol was used to dissolve it, in the vain hope that a
recrystallisation would be possible.
Rather than dissolve, the solid released en-masse from the glass in the form of a weird floating sheet of something !
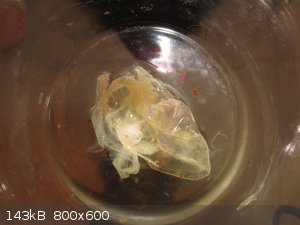
Removed and dried, this thing weighs 0.39g and is somewhat elastic, with surprising strength.

Another run of the hydration was started with 80ml of alpha-pinene and no acetone at all.
After 2.5 hrs refluxing the level of the water layer had not changed at all (calculation said it would, by ~6ml, as water would be consumed in the
reaction).
At all times the two layers remained separate.
Acetone was added and the mixture became homogenous.
This mixture will be refluxed for another attempt at recovering the product.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hmmm... Not looking good at all. Polymerisation due to these double bonds?
The 'a-word' is starting to depressingly spring to mind. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | Hmmm... Not looking good at all. Polymerisation due to these double bonds?
The 'a-word' is starting to depressingly spring to mind.  |
Which 'a-word' ?
aga
amateur
asshole
abomination
anathema
... all of which can apply !
[Edited on 3-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Abort. Sadly.
We may want to contemplate acquiring some alpha-terpineol.
[Edited on 3-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not a chance.
|
|
|
| Pages:
1
..
9
10
11
12
13
..
19 |