| Pages:
1
..
6
7
8
9
10
..
18 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  |
If companies like Aldrich sell hydrazine in glass bottles with sturdy caps, then I am inclined to think that the glass corrosiveness is not as bad as
people arte telling us. |
I had to dispose of my purchased glass bottle of concentrated nitric acid (70%) because the acid fumes rising up had caused the plastic screw cap to
completely fall apart after two years, despite the liquid never actually being in direct contact with the cap. One would think the chemical company
would know better, but apparently not.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I also had NH2-NH3OH (NH2-NH2.H2O, hydrazine monohydrate, 64% N2H4 solution) from Aldrich (it was more than 10 years ago)but it was in special PE
plastic bottles.
Maybe they have investigated the product and found out only concentrated hydrazine is dangerous to glass or maybe it is now a special treated glass or
coated glass?
I see what you mean, but the strenght of a base or of an acid is not an indicator of glass corrosiveness... as a proof HF, a medium weak acid, is
quite glass corrosive...all depends on the corrosion process involved in the corrosion 
Also it is possible the companies favourise the ageing of the consummers stock in a way they must refresh or use faster their product...so they buy
more...money money money...
[Edited on 18-5-2011 by PHILOU Zrealone]
[Edited on 18-5-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I mentioned in my last post, at the end of page 7 of this thread, that I had left some Hydrazine Sulfate out on a filter paper exposed to the open air
for 3 weeks.
I used it to make NaN3 and have just taken the dry yeild. I got a little less than a 50% yeild as usual. If the yeild is different than with fresh
Hydrazine Sulfate it is by less than 10%, I can't say exactly what the difference is because my process was not that precise.
To make a long story short, 3 weeks exposed to air at room temperature did not seem to make much difference to my Hydrazine Sulfate.
Edit:
Something else of interest I noticed. The last time I made NaN3 (over a week ago), I took the ~250mL of isopropyl alcohol with residual hydrazine and
isopropyl nitrite and put it in one of the empty plastic isopropyl alcohol bottles. I just noticed tonight that there is quite a large amount of NaN3
precipitate in the bottom.
I just added another 250mL tonight to the bottle. In a week or so, I am going to get the NaN3 out of there and weigh it. Who knows, with this I may be
up to the 70 or 80% yeild that Rosco says he gets (I think he said that somewhere).
Using the method I am using it takes so much isopropyl alcohol to dissolve the NaOH, that it makes for a very dilute solution and a sluggish reaction.
I was still getting precipitate forming after a week!
Here is a picture of the second precipitate from the first ~250mL. I started with 6.5g of HS, and collected ~1.5g of NaN3 from the first precipitate,
which I guess is really only about a 46% yeild. There does appear to be quite a bit more NaN3 formed though. BTW the bottle is a 500mL HDPE bottle.

[Edited on 19-5-2011 by Hennig Brand]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Just a quick correction. On the last page of this thread I stated that Fibrosis of the liver was reversible and that Cirrhosis was not. I was mostly
going on what somebody else told me who should have known. I recently asked a doctor about it and he said Fibrosis normally results in Cirrhosis, and
that it is Fatty Liver which is most often reversible. A few sources on the internet did indicate that Fibrosis can sometimes be reversible without
leaving persistant scarring, but most often it is not reversable.
Fatty Liver is caused by any "insult" to your liver, Infection/sickness, toxins (hydrazine) and possibly even obesity are all believed to contribute
to "Fatty Liver".
So on the last page when I mentioned Fibrosis, what I should have said was Fatty Liver (I think).
|
|
|
detonator
Harmless

Posts: 29
Registered: 12-4-2011
Location: Maoming City, Guangdong Province, China.
Member Is Offline
Mood: No Mood
|
|
Do not know whether the hydrazine and sodinm nitric can response,if possible,do not know whether it is production of azide?????? 
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
I just finished my first hydrazine sulfate synthesis following the RB procedure. The yields are not bad, but I’m a little bit worry, because the
crystals are still acidic even after the filtering and the alcohol washing. Is this normal, or I have to continued with the alcohol wash?
----pH: 1.3 (0.2M, aqueous)----
Well it's obvious now....
[Edited on 18-6-2011 by Jimbo Jones]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The hydrazine sulfate gotten by precipitation from an acidic solution using an excess of H2SO4 is the monohydrazine sulfate which is definitely an
acidic salt
and is far less soluble than the neutral salt which is the dihydrazine sulfate.
Hydrazine is a +1 base and associated covalently with a -2 acid H2SO4 you are going to net a -1 acidic compound something like an acid sulfate. Or
it could be thought of as the neutral dihydrazine salt associated with an acid of crystallization ....which is more likely the reality .....but for
simplification it
is simply by convention called the "mono"hydrazine sulfate .....and further
omitting the "mono" .....because it is the commonly supplied form that everyone understands and recognizes as "hydrazine sulfate".
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Yes, a salt formed from week base (the diluted hydrazine solution) and strong mineral acid. Member of this forum (Artem) sujested hydrazine nitrate
route from solution of Ca(OH)2 in which the hydrazine sulfate is converted to dihydrazine sulfate and then mixed with concentrated calcium or barium
nitrate solution. I think the low water solubility of barium nitrate will produce more pure product, because when you chill the solution the exess
barium nitrate will just precipate. A little bit messy, but the whole process is very simple and free from hydrazine fumes.
I'm planning to mix the hydrazine nitrate with aluminum powder and bind the whole mass with NM & NC jelly, which (I hope) will protect the nitrate
from moisture and LOD. NM is also a good choice of fuel too.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There are way better more interesting things to do with hydrazine as an intermediate, where it can ultimately contribute more energy in a derivative
product than some astrolite type mixture.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
I know that, but the hydrazine plastique above is cheap and easy to produce and will eliminate most of the troubles associated with the hydrazine
nitrate explosives. The need of strong confinement, good density and very strong booster. It’s not ordinary Astrolite based energetic, which means
low toxicity, better storage and nice molduble properties.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jimbo Jones  | " the hydrazine plastique above is cheap and easy to produce and will eliminate
most of the troubles associated with the hydrazine nitrate explosives." |
Really , how did you come by this knowledge ?
Hydrazine Nitrate + Nitromethane + Nitrocellulose = ?
Hydrazine Nitrate on it's own as a solid tends toward low detonation,
the overall variability in VOD and variable sensitivity as a liquid militates
against predictable results and stable storage when yet combined with
other materials of dubious consistency.
See Sickman
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
my post following
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
then artem
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
and PHILOU Zrealone
http://www.sciencemadness.org/talk/viewthread.php?tid=10058#...
Be aware also that a general rule of shock sensitive liquid explosives is that the
presence of minute bubbles makes it much more sensitive to much less of a shock.
In this condition concentrated Astrolite liquid is reportedly " as sensitive as ethylene
glycol dinitrate " that's a direct quote.
I'm reminded of another quote I read , written by Gerald Hurst , Cheif scientist
at the Atlas Powder company that developed Astrolite ,
" Several fellows discovered new ways to detonate it , but they're not around
to tell how they did it. Astrolite will blow up for it's own reasons and it is the
reasons you don't know that will kill you. Never try to make Astrolite of any kind
in glasssware unless , you're tired of living. "
Not unrelated
http://www.sciencemadness.org/talk/viewthread.php?tid=16612
________________________________________________
P.S.
Why is this discussion in an azide thread ?
.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
""Really , how did you come by this knowledge ?
Hydrazine Nitrate + Nitromethane + Nitrocellulose = ?""
Hi, what do you mean with this “how did you come by this knowledge”. The NM and the aluminum powder are good and stable choice of fuels. The NC is
simple energetic binder and will slow the evaporation of the NM & protect the HN crystals from moisture. A variation of the famous ANNMNC on
steroids. It’s close to mind, so what’s so special about it?
""Hydrazine Nitrate on it's own as a solid tends toward low detonation,
the overall variability in VOD and variable sensitivity as a liquid militates
against predictable results and stable storage when yet combined with
other materials of dubious consistency.""
I don’t have any previous experience with HN, but from what I have read the density, the good confinment and the strong booster are important to
insure high order detonation. I never used steel pipes or other metal container, so I chose the energetic plastic binder and aluminium powder which
are proven sensitizer in the different explosive formulations.
Originally posted by Artem:
"“This results are typical for unconfined and cardboard tubes charges at diameters up to ~30mm. For charges > 40mm VoD vs density continuously
increases. For example, figure 8.51km/s at 1.59g/cm3 was recieved for 2.5inch-charge (1th Symposium on Detonation, 22).
On my experience, in steel tubes and powerfull initiation HN gives exceptional brisance at diameter 20-25mm or more.”"
Am’ I missing something?
""Be aware also that a general rule of shock sensitive liquid explosives is that the
presence of minute bubbles makes it much more sensitive to much less of a shock.
In this condition concentrated Astrolite liquid is reportedly " as sensitive as ethylene
glycol dinitrate " that's a direct quote.""
It’s not a liquid explosive or Astrolite based composition. It’s plastique based on HN, NM, Al and NC.
""P.S.
Why is this discussion in an azide thread ?""
My first post was about the hydrazine sulfate process, I just wanted to share some additional thoughts. Sorry for that! In the future I’ll keep my
mouth shut!
[Edited on 21-6-2011 by Jimbo Jones]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Jimbo Jones
I can find no information whatever regarding blending Hydrazine Nitrate with Nitromethane.
The properties of the less reactive Ammonium Nitrate + Nitromethane are well studied and
the material composition is only prepared just prior to use as it is not stable in storage.
Nitromethane is somewhat sensitized by Aluminum more so by Nitric acid but particularly by
Hydrazine or its salts in amounts as low as 3 to 5 percent. Both Nitrocellulose and Nitromethane
are photolytically degradable , exacerbated by contaminants initially present and nitrogen oxide
accumulation subsequent to their formation. Hydrazine and Nitrogen oxides mutually decompose
producing more gas and moisture which promotes further decomposition and gas void formation
which acts to further sensitize the brew. All this contradicts your assertion which remains unfounded.
A related observation
http://www.sciencemadness.org/talk/viewthread.php?tid=16612&...
Physical & Explosion Characteristics of Hydrazine Nitrate
http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/1970001...
Quote , pdf page 18 , article page 13 " Compatibility Studies of HN With Various Materials "
" a frozen mixture containing equal proportions by volume of HN and Nitrogen Tetroxide initially at -100 ºC
showed that such mixtures react exothermically when the temperature is raised to -40 ºC."
- My comment - Not surprising since Hydrazine and Nitrogen Tetroxide is hypergolic rocket fuel. Having
Hydrazine in contact with nitrogen oxides is a bad idea generally.
Quote , on pdf page 19 , article page 14
" Medard ( 19 ) found that weakly nitrated explosives could be appreciably sensitized by a small amount of HN."
[19] Medard, Louis. Propriétés Explosives du Nitrate d' Hydrazine ( Explosive Properties of Hydrazine Nitrate )
Memorial du Poudres vol 34, 1952, pp. 147 - 157
Hard Start Phenomena in Hypergolic Engines
Volume III - Physical & Combustion Characteristics of Engine Residues
Also contains same parts excerpted in the report above
http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/1975000...
Attachment: Decomposition Mechanisms & Chemical Sensitization in Nitro, Nitamine, & Nitrate Explosives.pdf (645kB)
This file has been downloaded 1168 times
Found here on pdf page 187 , document page 1027
Proceedings of the International Symposium on Detonation (9th) Vol II
www.dtic.mil/dtic/tr/fulltext/u2/a247996.pdf
Ethylenediamine Dinitrate and Its Eutectic Mixtures
www.dtic.mil/dtic/tr/fulltext/u2/a131641.pdf
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Plain hydrazine and nitromethane are miscible, one would think hydrazine nitrate would also be soluble in nitromethane.
"With nitromethane, hydrazine forms an explosive salt."
"Nitromethane is strongly sensitized by hydrazine"
For some idea about the possible the reaction between hydrazine and nitromethane, you might read about the related reaction between ammonia
and nitromethane, which forms ammonium methazonate.
(2)NH4[+] [-]ON=CHCH=NO2[-]
https://sites.google.com/site/ecpreparation/methazonate-salt...
http://www.sciencemadness.org/talk/viewthread.php?tid=16118
http://www.sciencemadness.org/talk/viewthread.php?tid=1089
[Edited on 25-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Actually Franklyn, no one ever made valuable tests around with ANNMNC except Axt, so the storage stability is under VERY big question too. Years ago
I‘ve also made some tests with different AN/NM/NC % (just to hold more NM), but never tested any storage stability. After all AN is acidic by
itself, which obviously is not too good for the NC and soon or later will destroy the “plastique”. So ANNMNC is equally or less unstable to my
HNNMNC brew, but no one ever mentioned any possible danger.
Ok, from what I understand (thanks RED) the main problem will be the behaiver of the NC. NM will be sensited by the HN, that’s for sure, but aside
that this nitro paraffin and the aluminum powder are fairly stable and unreactive. NC on other hand don’t like semi soluble in water solvents like
NM, especially in combination with salts like HN. That’s why I think to skip the NC binder and will just use HN, NM and aluminum powder. If I’m on
the right track this will eliminate the need of strong confinement and booster, but will manage to keep very good speed and brisance.
P.S.
This is my last post in this thread.
<<<<All this contradicts your assertion which remains unfounded.
A related observation
http://www.sciencemadness.org/talk/viewthread.php?tid=16612&... >>>>
No one is perfect or protected from mistakes Franklyn. I’m not a chemist, but work very hard to fill the gaps.
[Edited on 26-6-2011 by Jimbo Jones]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This entire long thread has not been reviewed but I am sure that it has come up before the possibility of producing sodium azide in aqueous solution,
via nitrosation of semicarbazide, as a perhaps simpler alternative to the
reaction of free base hydrazine with isopropyl nitrite in alcohol containing sodium hydroxide.
There may be a workable method for doing the nitrosation of semicarbazide using sodium nitrite, and simply digesting the
aqueous reaction mixture at elevated temperature, which
reportedly will decompose the nitrosation product of semicarbazide, Carbamic Acid Azide, to Hydrazoic Acid, Ammonia, and CO2. There is only a brief
mention of this in Richter near the bottom of the page attached.
Attachment: Carbamic Acid Azide page 447.pdf (188kB)
This file has been downloaded 1027 times
[Edited on 26-6-2011 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
BTW, in case anyone wanted to know the result of letting the reaction mixture in the picture above sit for a week, here is a picture of 0.5 grams of
it (sodium azide) which I saved (the rest I used to make lead azide).
Notice that it took on a very pale yellow color, it still seems to work fine though.
I made a mistake in that I should have taken the yeild of the precipitate after the first 250mL had been in the bottle a week. I can however say that
I get approximately a 46% yeild by letting the reaction mixture set overnight ~12hours. I can also say that this yeild increases to ~70-75% (though
the second crop is a bit yellow in color), after letting the reaction mixture sit in a sealed bottle for about a week.
The 0.5g in the picture is just what I kept, the rest was used for making lead azide. Even though a little yellow, it made lead azide which seems to
be as good as any other I have made.

[Edited on 27-6-2011 by Hennig Brand]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
From www.sciencemadness.org/talk/viewthread.php?tid=1987&page...
Quote: Originally posted by Rosco Bodine  | I have an idea formaldehyde hydrazone may possibly be an interesting candidate for experiments towards producing azides or other possible compounds.
It seems like an interesting material that would have some interesting potential as a reagent for synthesis of various compounds.
|
Formaldehyde Hydrazone , CAS 6629-91-0
CH2:NNH2
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8112...
Lower Aliphatic Derivatives of Hydrazine
www.dtic.mil/dtic/tr/fulltext/u2/021177.pdf
One idea I just had is reacting it with nitric oxide will leave a free bond ,
would a six nitrogen chain form ?
2 CH2:N.NH2 + 2 NO => 2 H2O + H2C:N.N:N - N:N.N:CH2
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  |
One idea I just had is reacting it with nitric oxide will leave a free bond ,
would a six nitrogen chain form ?
2 CH2:N.NH2 + 2 NO => 2 H2O + H2C:N.N:N - N:N.N:CH2
|
Fairly sure the last intermediate would just decompose into diazomethane and nitrogen (N2), if not an explosion. After doing some research, apparently
hydrazine and nitric oxide spontaneously react together.
This article may be of interest:
http://www.sciencedirect.com/science/article/pii/S0040403907...
It basically says that (C6H5)NHN=C(CH3)2 reacts with nitric oxide (NO), in the presence of O2, to form
(C6H5)N=N--C(NO2)(CH3)2
[Edited on 16-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ AndersHoveland
Placing the bulk reagents in contact at ambient temperature is futile , given that
unsymmetrical dimethylhydarzine is hypergolically reactive with nitric oxide , the
methylene hydrazone would not be less so. Dilute very cold , even cryogenic
solution is called for , perhaps even done in the dark since nitric oxide is photo-
lytically catalysed. The hydrazone being basic will dissolve readily in sulfuric acid,
nitric oxide gas can be introduced into the space above the acid solution in a
closed reaction vessel gradually diffusing into and dissolved in the solution.
The supposed reaction then would precipitate the likely insoluble product desired.
The prospect of having a likely sensitive explosive detonate converting the acid
over it into mist requires exceptional precautions be observed.
The freezing point graph of varied concentration of H2SO4 is quite bizarre , varying
in no consistent way across it's range , attaining the lowest point of - 61 ºC at
approximately 35 % , and actually tends to supercool down to - 70 ºC.
Sulfuric acid freezing point, boiling point, density values
www.sulphuric-acid.com/techmanual/Properties/properties_acid...
Freezing point of Sulfuric acid solutions
www.unionbattery.com/images/2.6.pdf
Sufuric acid technical bulletin
www.sulcochemicals.com/Acid_Electronic_copy.doc
Freezing points SO3 + H2O system
www.generalchemical.com/assets/pdf/Sulfur_Trioxide_Water_Fre...
________________________________________________
A reaction scheme having more probable success would be to utilize Carbohydrazide
which having two hydrazino groups on the same carbonyl assures probable ring closure.
Nitric oxide ( depicted ) or even nitrous oxide can be employed to yield in such case an
eight nitrogen ring. Both derivations being quite acid could be recovered as salts or
further reacted say with formaldehyde.
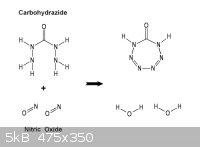
________________________________________________
While on the subject , it's worth mentioning that Guanidine may perhaps
undergo partial oxidation in this manner to yield Imidotetrazole. This is of
some interest as abundant prussic acid gas would result from detonation.
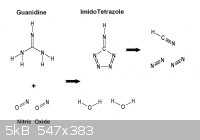
- Footnote -
HCN is endothermic having an enthalpy of formation of + 36 Kcal/ mol
so it is entropically disadvantaged to form in the chaos of a detonation ,
however it is not explosive and the alternative formation reduced carbon
and hydrogen are less favored.
[Edited on 18-7-2011 by franklyn]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I just do not know. As far as I know, such long chains of nitrogen have never been synthesized (unless stabilized by hydrocarbon groups on every few
nitrogen atoms. Aromatic rings have much more stability.
There have been many attempts to prepare/isolate "tetrazene" (N4H6), none of which have been successful.
Long linear chains of nitrogen atoms typically break down into diatomic nitrogen and radicals of nitrogen derivitives. I suspect that there might be
some way to prepare the type of compounds you are suggesting, but it would likely require dry ice/ liquid nitrogen temperatures. I am not aware of any
serious attempts at research into these highly unstable compounds. Typically, such reactions simply result in decomposition, liberating nitrogen gas,
or explosions. One would think that the reactions you are suggesting are straightforward enough that someone would have already tried them. In the
scientific literature, we much more often hear about the few successes than the many failures.
Nitric oxide may be produced by reaction of sodium nitrite with dilute hydrochloric acid, with ferrous sulfate, (FeSO4) also present to react with,
and prevent the escape of, the nitrogen dioxide. The reaction is basically:
(2)NaNO2 + (2)H2SO4 + (2)FeSO4 --> Fe2(SO4)3 + Na2SO4 + (2)H2O + NO
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
nitrosation of semicarbazide related
The prospect of an easier method for producing azides has caused mention at various times for years the nitrosation of semicarbazide or similar
compounds as a potentially convenient route, yet no really definitive method has yet been fully described. There are found many references made
somewhat as a brief notation or note in passing which suggests that a synthetic procedure having an azide as the intended product is possible, so it
seems odd that such an alternative synthetic route intending specifically to produce azides has not yet been described in all details as a dedicated
synthesis. Possibly such references do exist but are in different languages or in obscure older references and have never been surveyed and combined
in one English language summary procedure.
Axt mentioned this possible method six years ago in another thread http://www.sciencemadness.org/talk/viewthread.php?tid=6729
and the subject has been mentioned in variations several times in several different threads over the years, here and also at many other discussion
groups, with no followup reported which has brought more than little more scraps of information which could lead to formulating alternate syntheses
for azides, based upon nitrosation of hydrazine derivatives. There is something of an overlap for the chemistry involving azides with cyclized
products that are tetrazoles and triazoles and references more specifically about those materials also sometimes relates back to the more fundamental
azides. Attached is an article pertaining to Carbamoyl Azides which should be helpful, and the
reference #17 related to the nitrosation of semicarbazide
described in a German journal is additional reference which will be further attached here when it is obtained.
And additional general summary article concerning organic azides is here:
http://isites.harvard.edu/fs/docs/icb.topic208898.files/week...
Attachment: Carbamoyl Azides.pdf (709kB)
This file has been downloaded 1152 times
[Edited on 3-8-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Reference #17 Hofsommer and Pestemer article
The experimental part of the attached describes the reaction of nitrous acid and semicarbazide producing a 70% yield of Carbamyl Azide.
A translation of the relevant portion would be helpful if anyone may assist, thank you.
Attachment: Carbamyl Azide 70 per cent yield from Semicarbazide Hofsommer and Pestemer article.pdf (518kB)
This file has been downloaded 890 times
Über die Ultraviolett-Absorption und Konstitution von Tetrazenen aus Aminoguanidinsalzen
Ruth Hofsommer, Max Pestemer
Zeitschrift für Elektrochemie und angewandte physikalische Chemie
Volume 53, Issue 6, pages 383–387, Dezember 1949
DOI: 10.1002/bbpc.19490530612
Also back on page 3 and 4 of his thread S.C. Wack posted some attachments which are are scans of articles relating to oxidation of hydrazine sulfate
with H2O2 and nitrous acid and other oxidizers. Some more legible scans are now available and these are also attached as an update.
A New Synthesis of Hydronitric Acid (attached)
The Oxidation of Hydrazine (attached)
Attachment: A New Synthesis of Hydronitric Acid pages from JACS.pdf (196kB)
This file has been downloaded 809 times
Attachment: Pages from JACS Oxidation Of Hydrazine.pdf (769kB)
This file has been downloaded 1105 times
[Edited on 8-8-2011 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Potassium Azide
KN3 (Potassium Azide) has been discussed on the forum before, but not in much detail as far as I know. Going with the potassium salt can have its
advantages.
I am eventually going to make a large tilt tube manometer like Rosco uses for maintaining a safe pressure in the reaction vessel, and use methyl
alcohol as the reaction medium to make sodium azide, but building and using it is just not convienient right now. For now I have found that I can get
fairly good results without the transesterification issue with isopropyl alcohol and KOH in place of the NaOH.
I was having a hard time finding solubility information for the hydroxides in isopropyl alcohol. I could find lots of solubility information for the
hydroxides in methyl and ethyl alcohol, which showed KOH as having much superior solubility than NaOH in both alcohols. I figured it would be similar
for IPA as well.
I decided to try a synthesis and see how it went. I already knew from past NaN3 syntheses that it took over 250mL of IPA to dissolve 2g of NaOH (at
room temperature). For the same sized batch (moles of hydrazine), ~2.8g KOH is needed. I found that the 2.8g of KOH would easily dissolve in 150mL of
IPA, and maybe less. This change in reactant concentrations had a tremendous effect on the reaction kinetics. There may be other things which
contributed to the change in the reaction rate as well.
I started with 6.5g of homemade Hydrazine Sulfate in both experiments. I also did the Hydrazine freebasing with NaOH for both experiments.
Experiment 1
With the NaOH and 250+mL reaction mixture, I was getting approx. a 46% yield of NaN3 based on Hydrazine Sulfate after 12 hours. In order to get the
yield up to 70-75%, it took most of a week. I think 75 or 80% is about the max for this reaction anyway.
Experiment 2
With KOH and the 150mL reaction mixture things were much better. I left it for 12 hours, then decided to leave it another 12 (to tell the truth I don'
t think that much more precipitated after the first 12 hours though). So, after 24 hours I filtered and dried and was rewarded with a 76% yield of KN3
based on Hydrazine Sulfate. Potassium azide also precipitates in larger, easier to work with crystals than NaN3.
The heavy metal azides can be made just as easily from KN3 as NaN3, but the mass of KN3 used needs to be adjusted to compensate for the heavier
potassium cation in order to provide the same amount of N3 for the double displacement reaction.
Using methyl alcohol as the reaction medium would be much more efficient as far as materials are concerned, but this requires no special glassware at
all, or other apparatus.
Here are some pictures of my KN3 synthesis.
  
[Edited on 8-8-2011 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Interesting refinement there, basically using the method of Microtek through the point of the freebasing of the hydrazine, and then using KOH for the
third molar equivalent of the 3 total hydroxides. There is always about 10 to 15% residual H2O in KOH which can't be gotten out during ordinary
manufacture. When doing your calculations for stoichiometry if you weren't using some excess sufficient to account for the moisture then it could clip
the yield. But 76% is a very good yield so I am guessing you probably had a slight excess of base. It's touchy, because you want plenty of base to do
the job of neutralizing the hydrazoic but not so excessively basic as to decompose the nitrite ester as a competing reactant with the hydrazine. You
had to be in the right area get 76% yield.
|
|
|
| Pages:
1
..
6
7
8
9
10
..
18 |