| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I can't vouch for the quality of the purchased water. Here's all I could find:
http://www.ewg.org/health/report/BottledWater/Bottled-Water-...
The initial pH of the batch was 5 as indicated by pH paper so I didn't add any H2SO4.
My purpose in using spring water was that I believed it would contain essential minerals. I also felt that molasses would provide essential minerals
not provided by table sugar (sucrose).
I do know, however, that a friend has made ethanol successfully using just sucrose and Turbo yeast. I cleaned up his wash for him. The alcohol
content was ~10% IIRC.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by Magpie  | I can't vouch for the quality of the purchased water. Here's all I could find:
http://www.ewg.org/health/report/BottledWater/Bottled-Water-...
The initial pH of the batch was 5 as indicated by pH paper so I didn't add any H2SO4.
My purpose in using spring water was that I believed it would contain essential minerals. I also felt that molasses would provide essential minerals
not provided by table sugar (sucrose).
I do know, however, that a friend has made ethanol successfully using just sucrose and Turbo yeast. I cleaned up his wash for him. The alcohol
content was ~10% IIRC. |
Turbo yeast is often quoted as 18%-20%, 10% sounds about what I would expect, was this as a specific temperature at the still head or a range of
temperature?
Your water may well of been fine, if its bottled spring water rather than what the Scottish class as spring water then its mineral content would be
fine for short runs, I took spring literally as we have spring water here. Like the well water here or the bore hole water ours is incredibly soft
with a brown peat tannin tinge to it.
With out non tap water the CO2 via carbonic acid bounces the pH all over the place, our spring water has a TDS of around 120.
I hope the papers have been of some help so far.
Funny thing is with brewers they get rid of these side products and yet in some ways they worth more than the beer they make lol.
Natural Banana smell for soap via fermentation!! Soapers would pay for that 
Dont ask me, I only know enough to be dangerous
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Having slight issues download some papers in PDF so I 'dumping' some of the papers here that I will be using in doing this experiment, if you dont
mind that is. Let me know if I am cluttering your thread and I wll move to my ethanol thread.
Metabolic Responses of Saccharomyces cerevisiae to Valine and Ammonium Pulses during Four-Stage Continuous Wine Fermentations
PMCID: PMC3623169
p.s some of these might also be of interest to you, I will only post the free ones here, any paid for ones I will put in reference section
Dont ask me, I only know enough to be dangerous
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
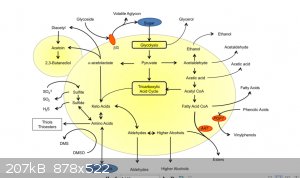
Here is a over simplified diagram of the different pathways for yeast in brewing. Although its incomplete and was taken from a brewing paper it shows
fairly well what pathways are available, reading the paper reveals the different worts etc and how they switch in and out the different paths, for
speed some of these experiments I am going to do on nutrient agar plates.
Rather than use the standard media I will use a range of different nutrients in the agar, that way we should be able to isolate pretty quickly the
nutrients and conditions to heavily favor the components we want the yeast to produce, I have also looked into the two main parent strains of lager
yeast. They are far more restricted than what the paper calls 'ALE' yeasts (Bakers yeast), Turborg yeast looked promising for a while but its a hybrid
of several yeast types and would be a pan to isolate pure cultures.
Once I get a plate that produces predominately what we are after I will mix up a solution with just the nutrients used for the plate and put in a
reactor to grow the yeast.
Then ferment 
Dont ask me, I only know enough to be dangerous
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Show me the isoamyl alcohol!
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
LOL I am going as fast as I can!!! gone back through ALL the work that was done to get rid of it, and have started backtracking from there, currently
have 16 plates and 32 tubes in the warmer.
Its looking likely I will have hydrogen by mid/end of next week, as soon as I have the gases I will test all the cultures for IA, those that show the
highest levels I will put into 400ml vessels and ferment according to the nutrient plate/tube they came from.
There is one tube where even with the dryer on the end of the vent the smell from it is really sickly sweet, Almost that kind of chloroform sweet or
maybe kind of almost ethyl acetate sweet.
Its hard to describe as I havent smelt many chemicals, but chloroform sprang to mind but much sweeter (in a sickly sense).
I dont like the smell at all, no sign of contamination on the lates though, they all seem clean.
Dont ask me, I only know enough to be dangerous
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by gsd  |
Fusel oil I obtained from a distillary is a dark foul smelling liquor. It is a cocktail of all lower alcohols (except methanol). It also contains
generous amount of water.
Gsd
|
Based on this excerpt from gsd's post upthread I'd say that those smells are encouraging.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Yes I think so, the problem is mostly which way you try and direct the respiration, looking at the picture above its easy to just say provide the
conditions that favor the pathway from pyruvate to acetaldehyde, and go that route, but thats the normal route that yeast or specifically normal fully
functioning (in the gene sense) Saccharomyces cerevisiae prefers to go, the actually by products at that point are mainly dictated by the sugar used.
But if you look closely the better route would be to favour the carboxyalic route, there are less factors involved if you can get the respiration of
the cell from the point of the pyruvate pathway to head off down that path, but again its even more important that the correct sugar is chosen because
it would need to be a fairly deficient nutrient broth.
The other major major problem is talking about this in terms of brewing, brewing is funny animal and everybody focuses on the yeast, but yeast only
play a part of the process in making beer.
If you read the paper that diagram was taken from, you see clearly how many micro organisms are actually involved, so when you see a brewery turning
out gallons of IA it could well have little to do with Saccharomyces cerevisiae, or Saccharomyces Turborg or whatever if its a lager.
I will attach the paper, its about brewing and the microbes generally involved. What I have been saying however is its absolutely possible to use
Saccharomyces cerevisiae on its own to produce IA, I have seen it and linked to papers that prove this, but its a biochemical process not a mix X
chemical with Y chemical and stir kind of thing.
The short way to all this is to jusy pick out the haploid yeast cells that show the trait you want and cross breed, or even shorter splice some e.coli
with the gene section you want. But no fun there and certainly no skill.
What I sugestead at the start of this thread should get you some of what you want, but if you want a refined and more reliable method you can repeat
time and again, then that takes a little time. I also have very limited funds so racking out 400 tubes of agar while very quick way to get to point B
isnt going to happen, and isnt needed. The frustration for me is my dad spent 4 weeks trying to get yeast not to produce IA!!! its was a total in the
arse.
So I have notes of great detail about how to avoid it, so its a back track process from them.
What worries me a little is the brewing connection, read the attached paper, it shows why in our case the two things are not connected, we are not
using grains and mashes as such, we are trying to use a pure yeast and single/mixed sugar combination with maybe a specific nutrient laced broth,
thats a long way from making beer
LG
Attachment: microbiology of brewing.pdf (1.5MB)
This file has been downloaded 482 times
Dont ask me, I only know enough to be dangerous
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I realize what a good challenge this is and I appreciate the time and effort you are putting into it. I also realize that the scope may be beyond
your funds and time available.
Chaim Weizmann developed an industrial process to produce butanol/acetone/ ethanol by fermentation of starch during WWI as acetone was in short supply
then and much needed for making cordite. IIRC it wasn't that easy and he was given a prestigious award for his work.
http://en.wikipedia.org/wiki/Acetone%E2%80%93butanol%E2%80%9...
[Edited on 27-11-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by Magpie  | I realize what a good challenge this is and I appreciate the time and effort you are putting into it. I also realize that the scope may be beyond
your funds and time available.
Chaim Weizmann discovered how to produce butanol/acetone/ ethanol by fermentation of starch during WWI as acetone was in short supply then and much
needed for making cordite. IIRC it wasn't that easy and he was given a prestigious award for his work.
http://en.wikipedia.org/wiki/Acetone%E2%80%93butanol%E2%80%9... |
LOL nah my post just sounds that way! Actually the moment you mentioned IA from yeast I realized how important it could be for me and my soap, I didnt
come across well thats all.
There is as much in this for me (maybe more) than for you, so its something I want to do, Yeah a bit frustrating not having huge funds to swamp it
with, but the main point is I want to make sure its efficient and repeatable, once we have done IA then we will have the parameters that allow us to
get the yeast to make others.
There is alot of literature based around this but we are also doing some unique work, most the work and papers are based on finding the pathways, not
on developing a process to actually manufacture these substances.
Thats why I wanted to do a paper on the other side of things.
What I didnt mention was, because of the distillery work I have access to grains etc they use, I am getting some live worts an some grains next week
from a distillery, I will try and isolate any microbes present.
I love this sort of thing, as much as I enjoy the chemistry, the biology side is more like what I am used to and have grown up with.
Dont ask me, I only know enough to be dangerous
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
The brewery that my parents work for is starting a distilling program soon (the giant, beautiful copper still arrived last week). I was reminded of
this thread when I was out there yesterday. They're a pretty large brewery, so I'm thinking that once they get this program up and running, there will
be plenty of fusel oil produced that they won't really care about. As such, I will try to get myself a bucket of it, and have a long-ish term project
to work on for a while!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | | They're a pretty large brewery, so I'm thinking that once they get this program up and running, there will be plenty of fusel oil produced that they
won't really care about. |
If it contains anything of value, they'll care about it, trust me. Someone's garbage is often someone else's gold.
But do try and get some samples, by all means...
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by zts16  | | They're a pretty large brewery, so I'm thinking that once they get this program up and running, there will be plenty of fusel oil produced that they
won't really care about. |
If it contains anything of value, they'll care about it, trust me. Someone's garbage is often someone else's gold.
But do try and get some samples, by all means... |
That is true, except that it is their garbage, my gold.

As far as I know currently, there are no plans to save it. Whatever I don't take goes off to water treatment. They aren't exactly the sort of company
that tries to turn every possible byproduct into a revenue stream. The only byproduct that they actually sell is their spent grain, but they go
through tons of it, and it is in high demand around here for use as cheap, healthy cattle feed. I couldn't see them charging anything for fusel
anytime soon.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I think it depends on how much of it they generate. It's mostly isoamyl alcohol which has quite a few uses, including the making of flavorings. They
would likely sell it to a distiller for purification - or at least give it away to reduce the BOD in their waste treatment process.
[Edited on 31-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm now remembering that the industrial alcohol producers who produce large quantities use continuous distillation columns. With these columns the
fusel oils tend to accumulate at a certain stage in the column so they are bled off at that point. These guys surely sell the fusel oil. I have a
paper on this somewhere.
With the drinking alcohol most stills are batch, especially for the current fad of craft distillers. Here the fusel oils stay in the "tails" as they
have higher molecular weight (lower volatility) than ethanol and water. So it is likely not economical to recover the them.
gsd can correct me on this if necessary.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I got several liters of tails from the still today (finally!) and I think I'll try some small scale runs to see how easily it can be fractionated. The
still operator said that it would still be approximately 55% ethanol, so that will be the first thing to remove. After that, we'll see!
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by zts16  | | They're a pretty large brewery, so I'm thinking that once they get this program up and running, there will be plenty of fusel oil produced that they
won't really care about. |
If it contains anything of value, they'll care about it, trust me. Someone's garbage is often someone else's gold.
But do try and get some samples, by all means... |
Not necessarily, biodiesel production follows a similar methodology. Non-commercial producers generally source their precursor fatty acids from fast
food chains etc.
Although I guess I'm stretching the definition of "valuable" a little here.
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah, they have no plans for the tails. What I don't take is dumped. I have about 5 liters currently to work with, though I could easily get more.
They are probably going to use the heads as a sanitizer, since it's pretty similar to denatured ethanol. I don't really have any interest in those
though, since all of the interesting stuff is in the tails. The 55% ethanol content is nice too. I just used up the last of the Everclear that I had.

One oversight on my part though is that I don't have a 24/40 column. I have a 14/20 one that would only work for tiny test runs, and a non-jointed one
that would be kind of a pain to work with, and is rather short. I won't be able to really get started with the full scale setup (2000 mL boiling
flask) until I get an appropriate column.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
When I distill moonshine, I usually do not even distill the tails, or feints. I turn the still off when it heats up to 92-94 C, leaving the fusel
alcohols in the still bottoms, which I ditch (literally, I pour them into a ditch). My typical batch consists only from the foreshots (solvent, fuel,
cleaning fluid, firestarter) and the main, drinkable middle.
My still bottoms are a smelly, watery liquid consisting from mostly water, three of four percent ethanol and some fusel alcohols.
|
|
|
| Pages:
1
2 |