| Pages:
1
2
3
..
30 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Moonshiners' 'Thumpers': Myth or Reality?
Being a homebrewer (but not a distiller) I've many times come across the concept of the so-called 'thumper', a device used by (mostly illegal) alcohol
distillers to alledgedly boost the EtOH content of their distillates.
This site here:
http://homedistiller.org/equip/designs/thumper
... makes a well meaning attempt at illustrating how a thumper is supposed to work its miracle.
The explanation remains in my view complete hogwash and I posit to the plenum that unheated thumpers simply cannot work and are part of moonshiners'
mythology.
A device into which the vapours from the primary still are condensed and then reboiled would of course be a different matter and NOT the type of
'thumper' that this thread is about. That set up would merely constitute two distillations in series and would indeed boost EtOH content, if large
enough in size.
I won't develop my arguments against an unheated thumper just yet, instead preferring to see what other members have to say about the subject, in
particular our resident distiller Zombie.
So, what say you all?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2662
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
If the steam from the first distillation heats the second pot, them it becomes a second stage of distillation. We used to have a reflux head that
acted somewhat like that for certain distillations.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dr.Bob  | | If the steam from the first distillation heats the second pot, them it becomes a second stage of distillation. |
You are merely parroting what the linked to site claims.
Not once have I come across a report that objectively compared EtOH yield with or without unheated thumper.
An unheated thumper loses much heat (if it didn't nothing could condense in it).
If this concept worked, doesn't it occur to you it would be exploited in other types of distillations?
[Edited on 15-2-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The writer seems like he's speaking from experience. But without reading this too closely it seems like bullshit, ie, it's violating thermodynamics.
I'm presuming the following:
1. The vapor from the still is at saturation, ie, not superheated.
2. The alcohol concentration in the thumper is equal to or less than that of the saturated vapor entering.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Magpie:
And not a measurement in sight!
Have a quick look at the excel spreadsheet he offers: pure, irrelevant BS.
The violation of Thermodynamics is clear: separation of EtOH and water costs energy, yet the thumper (unheated) LOSES energy.
[Edited on 15-2-2015 by blogfast25]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
It will act like a distillation stage, perhaps not too efficient but that's another matter. Between any liquid and gas en equilibrium will try to
form. Lets say the vapor concentration out of the boiler is 50% (roughly 9 wt% EtOH in the mash) and is condensed and kept at it's boiling point, then
what? A new equilibrium between the liquid and the gas will be established (75-80 wt% in the gas). There is no heating requirement, in fact there MUST
be some heat loss for this to occur. As long as the stage doesn't provide net heat (no liquid phase will form) or a full condensation occurs, some
distillation will happen.
Actually:
Forget that, this would best be described as "fractional condensation". Unless the distillate from the thumper is returned to the first stage the net
separation is given by the composition of the vapor out of the first stage. The result will be two fractions, one lower than the vapor and one higher.
If the condensate from the thumper is returned to the still a full distillation step will occur, and this will require extra energy.
[Edited on 16-2-15 by Fulmen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Fulmen:
How do you explain that to increase the EtOH content in the vapour phase leaving the thumper energy MUST be used (as with any separation process it is
endothermic), yet no energy is added to the thumper (quite the contrary)?
Have you seen some of these setups? How anyone can believe these would boost EtOH is truly beyond me. It smacks a lot of the old 'HOH' scam, to be
honest.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
What spreadsheet?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Start distilling with an empty thumper. Vapour comes over at T, with mole fraction x of EtOH.
Condensate starts forming in the thumper, at mole fraction x of EtOH and the thumper starts heating up slowly until temperature is roughly T.
Any vapour that comes off the thumper then also has mole fraction x of EtOH.
'Nothing to see here', as they say.
[Edited on 16-2-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Look at the page I linked to in the opening post: he offers a spreadsheet download about half way down the page: thumper.xls it is called.
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Perhaps if you put a fractionating column on the OUTPUT of the thumper, you'd get a more concentrated vapor. But then you could just put one on the
still and forget the thumper altogether. The truth is, that if the alcohol solution in the thumper is less concentrated than the vapor, there will be
loss. To achieve the maximum "azeotrope efficiency," one would need to use a massive column, and the still pictured in the linked website shows no
such thing.
Fear is what you get when caution wasn't enough.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Blogfast: I stand corrected, I didn't read the description close enough.
|
|
|
Luke
Harmless

Posts: 20
Registered: 25-9-2014
Location: Australia
Member Is Offline
Mood: No Mood
|
|
it works at boosting alcohol content. Source, i've seen it work.
Its really loud though. The one i saw was using metal, maybe wood would be quieter.
Also you dont start with an empty thumper, you usually fill it with some alchol before you start.
Another thing to mention is besides claiming to increase final abv it cleans the spirit up a bit. So even if it didnt boost abv you'd still do it for
the better flavour profile.
maybe it works because the heat required to boil the higher abv liquid in the thumper is less than the lower abv steam coming in. Water vapour can
carry much more energy than alcohol vapour.
[Edited on 16-2-2015 by Luke]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
In what way have you "seen it work"? Did anyone compare EtOH content with and without thumper? Have any numbers? Advocates of HOH scams also claim
they've seen it work, w/o of course presenting the slightest shred of evidence.
The rest of your comment is unscientific bullcr*p.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm going to have to reset my security setting on my computer before I can look at that spreadsheet.
From another website (kgb):
Well now, if "steam is run through it" then it is indeed another still. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Let me see if I can answer this correctly.
Take a wash containing approx 10% EToH, and split it between two containers. Pot, and thumper. Mass wise about 70% in the pot, and 30% in the thumper.
Therefore there is Much more thermal energy in the pot.
The primary (pot) will boil at approx 190*f due to the EToH boiling point of 172.6*f, and the water boiling point of 212*f..
What is coming out of the primary pot is mostly EToH vapor, and very little water vapor. Lets say for arguments sake about 80 / 20 or 160 proof
Alcohol.
This is the part that I will mess up the explanation but basically the EToH carries a (+) rating on energy. This means it carries more stored energy
than it took to create the vapor. (see attached)
This excess energy in turn is used to heat / vaporize the liquid in the thumper, and the small amount of carried over water from the pot does
condense, and remain in the thumper.
The rhumper will exponentially be gaining a higher percentage of EToH from the pot, and a smaller amount will be leaving the thumper over any given
period of time. So the increasing EToH to water ratio is actually lowering the boiling point in the thumper, and the excess energy contained in the
pots EToH vapor has less, and less work to do to vaporize what is in the thumper.
The process becomes MORE efficient as the run continues, and you actually have to reduce the input power to the boiler or the run will speed up
stripping more water vapor out of the thumper.
Distillers want to avoid this due to ester compounds, and organic oils that will be vaporized, and carried over to the finished product.
I hope I explained this well enough to allow you to research the exact process or mechanism that allows it to work ie: EToH excess energy.
At least this is what I heard... I stay far away from crime things.
(note)
Abstract
The vapor pressures of (ethanol + glycerol) and (water + glycerol) binary mixtures were measured by means of two static devices at temperatures
between (273 and 353 (or 363)) K. The data were correlated with the Antoine equation. From these data, excess Gibbs free energy functions (GE) were
calculated for several constant temperatures and fitted to a fourth-order Redlich–Kister equation using the Barker method. The (ethanol + glycerol)
binary system exhibits positive deviations in GE where for the (water + glycerol) mixture, the GE is negative for all temperatures investigated over
the whole composition. Additionally, the NRTL, UNIQUAC and Modified UNIFAC (Do) models have been used for the correlation or prediction of the total
pressure.
I had to edit to add that this effect can be "daisy chained" until the thermal losses to the environment overcome the (+) energy contained in the EToH
vapor.
Some "shinners" will run up to three insulated thumpers, and reach azeotrope on the finished product.
[Edited on 16-2-2015 by Zombie]
[Edited on 16-2-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Awesomeness  | | To achieve the maximum "azeotrope efficiency," one would need to use a massive column, and the still pictured in the linked website shows no such
thing. |
That's not what we're discussing here.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zombie  | This is the part that I will mess up the explanation but basically the EToH carries a (+) rating on energy. This means it carries more stored energy
than it took to create the vapor. (see attached)
This excess energy in turn is used to heat / vaporize the liquid in the thumper, and the small amount of carried over water from the pot does
condense, and remain in the thumper.
|
Complete and unadulterated NONSENSE. It carries precisely the amount of energy you put into it: Principle of Conservation of
Energy.
Next we'll be talking perpetual motion machines! 
Still, Zomb, thanks for making my point for me! 
[Edited on 16-2-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
I'm going to have to reset my security setting on my computer before I can look at that spreadsheet.
|
I downloaded it and it was kosher.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by Magpie  |
I'm going to have to reset my security setting on my computer before I can look at that spreadsheet.
From another website (kgb):
Well now, if "steam is run through it" then it is indeed another still. 
|
Both statements here are correct. Thumpers got their name due to the vapor coming into them below the level of the liquid contained inside. The
resulting bubbling inside a wooden barrel has the effect of a hammering sound. It starts off pretty intense but as the temps equalize it is lees
pronounced.
The thumper is indeed a secondary distillation process just as I explained in my above post.
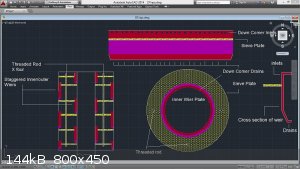
The statement on a"Massive column" being needed for azeotrope is no longer applicable. Modern distillers are running 4 foot tall x 4 inch round,
plated columns using either sieve plates or bubble caps to create hundreds or thousands of sources for vapor / liquid interaction. This is combined
with Reflux inducing condensers on the top of the column to make the process almost infinite, and fractional stabilization can be reach, and
maintained in very short amounts of time..
I have designed a 6 inch x 3 foot outer column, containing a 3 inch by 5 foot inner column (concentric) that combines the idea, and function of a
thumper with the efficiency of a plated column.
Every drop of EToH produced is azeotrope
[Edited on 16-2-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You haven't explained a goddamn thing. Instead you've violated the principle of energy conservation! 
A false premise can never lead to a correct conclusion.
[Edited on 16-2-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm taking that statement about steam as being an outside source of steam, not the vapor from the still.
Does a thumper have a steam coil or is it just a pot?
[Edited on 16-2-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Zombie  | This is the part that I will mess up the explanation but basically the EToH carries a (+) rating on energy. This means it carries more stored energy
than it took to create the vapor. (see attached)
This excess energy in turn is used to heat / vaporize the liquid in the thumper, and the small amount of carried over water from the pot does
condense, and remain in the thumper.
|
Complete and unadulterated NONSENSE. It carries precisely the amount of energy you put into it: Principle of Conservation of
Energy.
Next we'll be talking perpetual motion machines! 
Still, Zomb, thanks for making my point for me! 
[Edited on 16-2-2015 by blogfast25] |
I told you I would not explain it correctly.
Let's try again.
The vapor carried is enough to induce boiling in the thumper. The concentration of EToH increases faster than the the amount of water introduced,
therefore the boiling point in the thumper is reducing while the boiling point in the boiler is increasing.
This in turn creates the effect of carrying more energy than is required into the thumper.
Thus what Bert stated about introducing heat to the thumper is negated due to the excess energy introduced as the run progresses.
It can / will cause a runaway reaction where unwanted compounds begin to carry out of the thumper.
Maybe this is a better way to explain it.
[Edited on 16-2-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No, it isn't: it's the same nonsense re-hashed, 'complexified' w/o any elucidation.
Where in that beautiful design on the right is the thumper?
[Edited on 16-2-2015 by blogfast25]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by Magpie  | I'm taking that statement about steam as being an outside source of steam, not the vapor from the still.
Does a thumper have a steam coil or is it just a pot?
[Edited on 16-2-2015 by Magpie] |
It's just a pot. The steam is the water vapor carried over from the boiler into the thumper.
at the begining or "Boil up" it is a 50 / 50 proposition, and the pot has to be brought to a hard boil. As the EToH in the thumper increases the
boiler power is MORE than enough to continue the process, and eventually must be reduced.
Old school hillbillies figured this out on a second grade education.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
| Pages:
1
2
3
..
30 |