| Pages:
1
..
23
24
25
26
27
..
30 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | | I believe that you got false readings because of the presence of contaminants (e.g ketones, other alcohols, whatever) that skew the result, so-called
matrix effects in analytical chemistry. You would need to calibrate with those contaminants present in order to get accurate readings, but that is off
course not practical, but just saying. |
If the problem is contaminants that skew the readings (and it seems very likely) then the solution is to use pure EtOH/water mixtures.
Extraordinary claims require extraordinary evidence. Claiming "I did this (and a few of my mates did so too)" just isn't near enough good.
|
|
|
manganese
Harmless

Posts: 1
Registered: 6-3-2015
Member Is Offline
Mood: No Mood
|
|
Dear Mr blogfast25, yiu are right extraordinary claims require extraordinary evidence. and just tall talk isnt the answer, one should be able to
deliver what one claims and commits .
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by deltaH  | | I believe that you got false readings because of the presence of contaminants (e.g ketones, other alcohols, whatever) that skew the result, so-called
matrix effects in analytical chemistry. You would need to calibrate with those contaminants present in order to get accurate readings, but that is off
course not practical, but just saying. |
If the problem is contaminants that skew the readings (and it seems very likely) then the solution is to use pure EtOH/water mixtures.
Extraordinary claims require extraordinary evidence. Claiming "I did this (and a few of my mates did so too)" just isn't near enough good.
|
I don't think you will be able to accomplish this from a single stage mash distillation. It is very likely there are significant amounts of byproduct
that were co-distilled with the ethanol and water mixture. Unless you are fractionating, I don't see how there couldn't also be a few other things in
the distillate. Significant as in enough to skew readings.
Is it also likely that one of these contaminants could contribute to surpassing the azeotrope? If what Zombie says about watching his hydrometer
dropping back to azeotrope is true, what could account for this behavior? What else could be absorbing water, or could have caused the ethanol to be
in higher quantity?
As with any application of systems theory, there are usually far many more variables than is obvious or that we can actually account for.
I start to think about when you are attempting to break azeotrope by distilling with a desiccating agent, such as calcium oxide, and it hit me
that it could be possible that something else in the mixture could contribute to the same end, by messing with the dynamics of the mixture.
[Edited on 20-3-2015 by Loptr]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
At some point we have to take a man at his word, if Mr. Zombie says he has them calibrated it's good enough for me. Unless he's deliberately lying (in
which case this whole debate is worthless) I don't see the point in second-guessing this.
That being said, his claims of breaking the azeo and the actual numbers are still up for grabs as far as I'm concerned. Having spent a couple of
decades in a lab I know that calibrated equipment is only half the analysis, and in my experience hydrometers can be tricky.
Have anybody here ever participated in a ring test? Reviewing the numbers from other labs shows just exactly how imperfect analytical chemistry can
be, you'd be surprised how much variation that can be tolerated.
So let me ask you this question Zombie: Have you tested your method on any known standards? And since your equipment seems to be based on
proof strength, what was the actual reading in proof?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Loptr  | | when you are attempting to break azeotrope by distilling with a desiccating agent, such as calcium oxide, and it hit me |
Perhaps that is a 'secret', and the billies put some sort of desiccating agent or third/fourth/fifth agent in there that can affect or participate in
the azeotrope.
Ah crap. It's all Guessing.
@Zombie: please don't take the lack of belief in your claims as anything other than Scientific minds refusing to Believe anything at all without hard
data to back up that belief.
Nobody is saying you're lying.
The consensus is currently that your claims are groundless, in that your experience has not been replicated by at least two others, following the same
procedures, and arriving at near enough the same results, with hard data (measurements) to prove it.
Seems to me that a thumper works as advertised, near as damnit, yet i may have just got lucky, measured the wrong thing, gotten drunk while
experimenting, read the refractometer upside down etc etc.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fulmen  | Have anybody here ever participated in a ring test? Reviewing the numbers from other labs shows just exactly how imperfect analytical chemistry can
be, you'd be surprised how much variation that can be tolerated.
|
Yes, on one intra-lab comparison of methods. It was a humbling experience but not surprising.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by aga  |
The FeedStock for all experiments was 500ml of cheap 37.5 w% Vodka diluted with DIW to give 1875ml of 10 w% EtOH/Water (as measured by the
refractometer).
|
Let's keep in mind what the feedstock was. It's not Sigma 4nines absolute ethanol but it's certainly not corn wash either. My point being, how much
extraneous organics (aldehydes, ketones, esters, other alcohols, etc) can we really expect to find in cheap vodka? Enough to cause the azeotrope to
disappear? I'm doubtful.
But that is a separate issue from Zombie's claims with his own equipment where I presume his feed was a grain wash.
[Edited on 20-3-2015 by Magpie]
[Edited on 20-3-2015 by Magpie]
[Edited on 21-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The feedstock certainly has aromatics added - it smells of things other than EtOH.
Only 1 of each experiment was done, and by the Forum Drunkard, so the results should be regarded as a bit of additional information rather than Gospel
truth.
More experiments to follow, hopefully with more useful data.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
As it was commercial vodka I'm sure there is data on the actual purity available. Certainly someone has run a bunch of vodkas through a GC before?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If only some member had a Gas Chromatograph at their disposal !
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
This should provide some data:
http://www.google.no/url?sa=t&rct=j&q=&esrc=s&am...
We're not banging rocks together here. We know how to put a man back together.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Initial verification of the refractometer with various dilutions of said vodka showed good correlation between predicted and observed ABV values,
which tentatively suggests that contaminants did not play a major part in aga's experiments and their outcomes. Invoking any unknown
interactions between contaminants and EtOH seems a little far fetched at this point.
For further elimination of any non-EtOH, non-water components, a batch of vodka could be distilled (with a Vigreux column for instance), retaining
only the middle fraction of the azeotropic distillate as new feedstock of any future thumper experiments.
Thanks for that link, Fulmen.
[Edited on 21-3-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
FYI, Figure 3 of Fulmen's linked document indicates 1350 ppm of non-ethanol organics in a typical "alcoholic beverage," or 0.135%.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Suck-back Problem Solved.
It really is simply a matter of keeping the output of the boiling pot going at a high enough rate.
Using a 250ml RBF as the boiling pot allowed the transfer pipe to be 30% shorter.
Adding Al foil insulation to the whole thing also helps, mostly on the boiling pot.
Anyway, this rig now thumps properly with no suck-back, and exhibits some strange behaviour.
Data will be shared as soon as some sense can be made of it.
  
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Although not finished, I thought I would post some preliminary results from my trials this weekend. Since I couldn't justify buying an alcohol
refractometer that I would probably only use once, I opted for a little less accuracy and measured density of the distillate using a graduated
cylinder and a scale. I used this table to determine ethanol concentration from density.
The feedstock was a wash prepared from brown sugar, molasses and water, fermented to about 4.6 ABV, or about 3.4 % ethanol by weight (assuming density
of ethanol as 0.785 g/mL).
The boiling pot was a 2000 mL round bottom flask, the thumper was a 500 mL three-neck round bottom flask. The pot was connected to the thumper via a
rubber septum, a 6 mm o.d. x 4 mm i.d. copper tube running to the bottom of the thumper, and a PTFE thermometer adapter in the center neck. One side
neck was plugged with a rubber septum, and the other housed a distillation head with a thermometer. The still head was connected to a Liebig
condenser. Heating was done with a Corning hotplate/stirrer. The still head was insulated with aluminum foil for all experiments. No suckback ever
occurred until distillation was finished and the heat was removed.
Practice Run:1500 mL wash in the pot, 250 mL in thumper: Large (~3 to 5 mm diameter) bubbles were released in the thumper as
dissolved carbon dioxide was released from the wash, then the bubbles were much smaller (0.5 to 2 mm) as the pot began to boil in earnest. The liquid
level in the thumper rose as vapor from the pot came over, then bubbles increased in size again as distillate began to come through the condenser.
The temperature in the head fluctuated between 82-87 degrees C until adjusted to 81-83 degrees. The distillation was stopped the thumper was full. A
total of 21 mL of distillate were collected, with a density of 0.857 g/mL, corresponding to about 71% to 73%
ethanol by weight (not correcting for other volatiles).
Run 2: 1500 mL pot, 200 mL in thumper. The pot was heated slowly and carefully, in order to watch the temperature in the still head
slowly climb. ~0.9 mL of distillate were collected below 80 degrees C. This was discarded. The thumper filled as before; in order to prolong the
run, the thumper was siphoned back down to ~250 mL once it had filled. At this point a total of 30 mL of distillate had come over. Not counting the
initial low-boiling discard, two fractions were collected, each of 20 mL.
F1: d = 0.852 at 25 degrees C, [EtOH] = ~74% by weight.
F2: d = 0.841 at 25 degrees C, [EtOH] = ~80% by weight.
Simple distillation, practice run: Setup was for standard distillation, with the usual glass: 2000 mL pot, connected to the still
head with thermometer, which was in turn connected to the Liebig condenser. 1500 mL wash was added to the pot and heated. It was more difficult to
control the temperature in the head here than with the thumper. After distillation began, the first ~ 1 mL was discarded as before; the temperature
went up over a few minutes to ~92 degrees C, and 5 mL distilled over before the heat was removed and the setup was allowed to cool a little before
trying again with gentler heating. This time, the lowest temperature achieved while still maintaining a slow distillation (about 3 to 5 drops per
minute) was 84 degrees. The temperature slowly rose to 92 degrees over the course of the distillation. Two 16 mL fractions were collected.
F1: d = 0.874 at 25 degrees C, [EtOH] = ~66% by weight
F2: d = 0.889 at 25 degrees C, [EtOH] = ~60% by weight
I am going to continue the experiment next weekend, when I have time again, the simple distillation could definitely stand to have better temperature
control. The measurements are fairly crude, but they were all done the same way, with the same equipment. I am open to questions, comments and
suggestions on the experimental protocol, within the limits of my materials and equipment.
[Edited on 22-3-2015 by Crowfjord]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice one Crowfjord.
Photos !
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
The lens is scratched, so the photos are not great quality, but here are a couple of the thumper setup.
 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Brill. Thanks.
Edit :
The transfer pipe goes down to near the bottom of the thumper right ?
[Edited on 22-3-2015 by aga]
[Edited on 22-3-2015 by aga]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Yes, it does. There is only ~1 mm of space between the bottom of the copper tube and and the bottom of the 500 mL "thumper" flask.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thumper/No Thumper distillation comparison
I’m reporting here the conditions and conclusions of Aga’s experiments with thumper/no thumper stillpot distillations using the apparatus
described in the posts above (in near-identical conditions for both runs), based on raw data he supplied me over the weekend.
Initial suck-back problems from the thumper were resolved by increasing distillation speed somewhat. A short video of the vapour passing through the
thumper liquid can be found at http://www.vxwifi.co.uk/thump.MOV (during a separate test run).
The thumper experiment was run with an initially room temperature and empty thumper which gradually filled up during distillation, until vapour
started bubbling through it.
During distillation, distillate samples of approx.1.5 ml were collected and weighed. In most cases these had to be diluted with about the same amount
of water (to allow measurement with the refractometer). This amount of water was also weighed. From the diluted ABV values, both weights and a density
correction factor (the densities of the distillate and water are not identical), the actual distillate ABV was then calculated.
The data also allowed calculation of distillation rates but considerable data scatter made drawing significant conclusions difficult. Broadly speaking
distillation rates in both experiments were comparable.
Initially the stillpot in both distillations held 200 ml of 9 ABV % EtOH (diluted Vodka, then measured).
Basic data:
With thumper: distillate started coming over about 22 minutes after start of heating, In total about 23 ml of distillate was collected with an
average (calculated) ABV % of about 61. Remaining pot and thumper liquids at the end of the distillation were essentially EtOH free. Mass balances in
EtOH tallied satisfactorily.
Without thumper: distillate started coming over about 14 minutes after start of heating, In total about 23 ml of distillate was collected with
an average (calculated) ABV % of about 48. This enrichment is very roughly in accordance with 1 theoretical plate.
The most important metric is the ABV (%) vs Distillate Volume graphs for both distillations below.
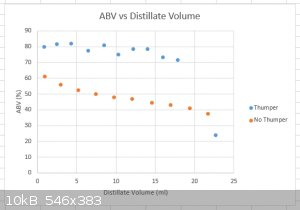
Conclusion:
The improved EtOH enrichment during the thumper run is highly significant.
Acknowledgement: I want to thank Aga again for his work and persistence in completing this experiment, which to an unsuspecting
onlooker may seem far simpler than it actually is. Without them, this investigation would not have been possible.
[Edited on 30-3-2015 by blogfast25]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Nice work, gentlemen! Well done, both of you!
I did a couple more simple distillation runs this weekend, and our results seem to agree with one another, despite my crude quantitation. I was never
able to get a distillate richer than 66%(w/w) ethanol with simple distillation, and that was with distillate passing over excruiatingly slowly.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Brilliant! Fantastic work aga, and nice job putting it all together, blogfast.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Crowfjord:
Nice work!
Did you calculate the amount of sugar in the ‘mash’ to give about 5 % ABV after fermentation?
Density measurements are fine of course. It could be informative to repeat your procedure several times with distilled or deionised water to get an
idea of accuracy/repeatability. Wiki lists the EtOH density as 0.789 g/cm<sup>3</sup>, not 0.785 BTW.
Tentatively it appears your thumper contributes less to enrichment than aga’s. I wonder, again tentatively, whether starting with an empty thumper
gives an advantage here.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The thumper liquid is never cold if you start with an empty thumper, so perhaps that has a beneficial effect.
It'd be an idea to try it with a heated thumper too, although i suspect the temperature of the input from the boiling pot is important as well (never
much more than EtOH's B.P.)
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
My mash had about 4.6% ABV, going from gravity to estimate change in sugars. I have checked my graduated cylinder with distilled water before, and
found it to be accurate to within 0.5 mL, but I should do it again to make sure.
The density I used came from the ethanol-water density chart I linked above, for ethanol at 25 degrees C, which was the temperature at which I took
all of my measurements, give or take about 2 degrees.
I suspect that starting with an empty thumper would result in greater enrichment, at least early in the run. I still have some wash left, so I might
give it a go next weekend.
As mentioned up thread, thumper volume relative to that of the pot probably contributes to the effects on the concentration of the distillate. That,
and our different starting ethanol concentrations probably contributed to our differing enrichment rates.
|
|
|
| Pages:
1
..
23
24
25
26
27
..
30 |