| Pages:
1
..
19
20
21
22
23
..
33 |
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I'll do a post when I get home later regarding the possible isomers (enantiomers included) and their respective IUPAC names.
And aga . . . you know damn well why phenol is more acidic than non-aromatic alcohols! How many times have I explicitly linked
electron delocalization to acidity and ease of proton removal? This is why I keep stressing resonance!!!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Eeeeeeek !
These last two posts have triggered my canine relflex.
I need some Basket time with my paws over my eyes.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Alrighty: 'permission to snooze', Big Paws!
When you wakey wakey when the Big Fireball rises above the Land again and you're having your first Crunchies, remember that Old Grumps' spiel about
hexachlorostannate is not a genesis story of that ion, only an explanation of its actual structure... 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Erratum:
On the colours of f-block salts, I wrote "4d" where of course it should have read 4f and "5d" where of course that should have read
5f. The correct text is below.
Quote: Originally posted by blogfast25  |
Important corollary:
f block elements are also often colourful for similar reasons.
The 4f orbitals also split into two groups according to orientation and thus slightly different energy levels, in a ligand field. An incompletely
filled 4f orbital thus also gives rise to transitions with emissions in the VIS part of the electromagnetic spectrum. Most (+3) lanthanides show
colour, as do many actinides due to part-filled 5f orbitals.
|
Mea culpa.
[Edited on 24-11-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
As promised, here are all the possible position and skeletal isomers of 2-pentanol, complete with stereochemistry and correct IUPAC nomenclature.
Itself included, there are a total of eight possible alcohols from 2-pentanol: three of them chiral and the rest achiral, giving rise to a total of 11
different molecules all together. I would like to point out that there are technically other isomers that are also possible from 2-pentanol, which are
functional isomers like 1-ethoxypropane, 1-methoxybutane, 2-methoxybutane, 2-methoxy-2-methylpropane and so on; however, these isomers are ethers and
not alcohols, so I didn't bother to actually draw them.
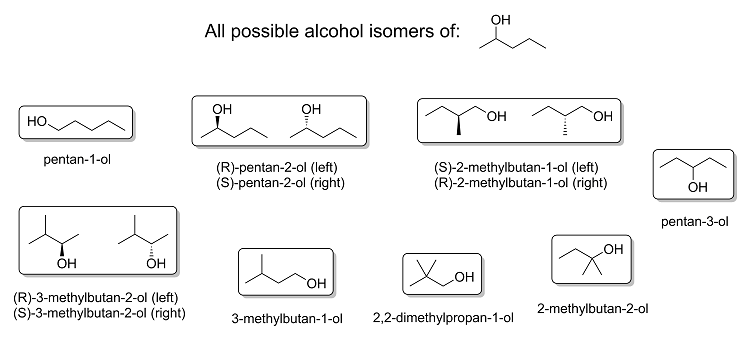
And in the unlikely event that anyone's interested, here's a much higher quality version of the image above in .png format and high resolution:
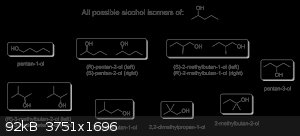
[Edited on 11-24-2015 by Darkstar]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks, Darkstar.
To try and give aga a bit of closure re. hexachlorostannate let's look at a proposed formation reaction mechanism of that ion.
For those in possession of the required chemicals the easiest synthesis is as follows. Cold pure SnCl<sub>4</sub> is added to a
stoichiometric excess of cold 37 w% HCl with intense stirring. The overall reaction equation is:
2 HCl(aq) + SnCl<sub>4</sub>(l) < === > H<sub>2</sub>SnCl<sub>6</sub>(aq)
The excess HCl serves to push the equilibrium fully to the right and prevent ligand substitution by OH<sup>-</sup>(aq) (discussed above).
H<sub>2</sub>SnCl<sub>6</sub> is a strong acid (its salts react neutrally) and thus more or less completely dissociated in
water.
Now let's look at the structure of SnCl<sub>4</sub>. It's the same structure as methane or CCl<sub>4</sub>: Sn's outer atomic
orbitals containing 4 electrons in total, hybridise to four sp<sup>3</sup> orbitals each containing 1 unpaired electron. These
sp<sup>3</sup> each then interact with 1 lone electron in the outer shell of a chlorine atom, to form SnCl<sub>4</sub> which
like its methane analogue is a tetrahedron.
So how do we get from SnCl<sub>4</sub> to SnCl<sub>6</sub><sup>2-</sup>? Obviously two choride ions have
to be added.
Successful three-species collisions are very rare for obvious reasons so it's reasonable to expect the addition process to be a two step process. A
first successful collision between a SnCl<sub>4</sub> and a chloride ion would give rise to an intermediate and probably excited
SnCl<sub>5</sub><sup>-</sup>. A second successful collision with a second chloride ion would yield
SnCl<sub>6</sub><sup>2-</sup>, probably in an excited state. Mild rearrangement of the formed MOs to minimise their mutual
electrostatic repulsion then leads to the octahedral structure of ground state SnCl<sub>6</sub><sup>2-</sup> with electronic
structure pointed out in the higher post.
An interesting point to note here is that chloride ions here play the part of Lewis bases while SnCl<sub>4</sub> plays the part
of a Lewis acid. SnCl<sub>4</sub> is indeed used as catalyst in Friedel-Crafts addition reactions.
The reaction product of a Lewis base and a Lewis acid, like SnCl<sub>6</sub><sup>2-</sup>, is known as a Lewis
adduct.
[Edited on 24-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thank you both for the tummy tickles.
I feel like such a Good Boy that i've got to wag my tail for a while.
Hope i don't get arrested again like last time.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga, for my 'next trick': do you know what amphoterism is?
[Edited on 25-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
ISTR that amphoteric is to do with a closed system with nothing going in/out.
In other words, No, i dont know.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Erm... no, that's an adiabatic system!
Ligand Exchange Theory: Explaining Amphoterism
We should by now be familiar with the concept of coordination complexes and their structure. A general coordination complex can be written as:
ML<sub>n</sub><sup>z</sup>
Where M is the central metal atom, L the ligand, n the coordination number and z (positive or negative) the overall charge of the complex ion.
The formation reaction for two generic complexes, ML<sub>n</sub> and MN<sub>n</sub> (for ease of notation the overall charges
and state symbols are not shown) are shown below:
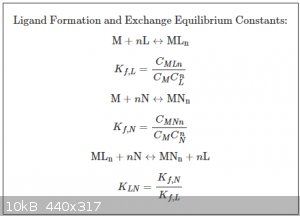
The K<sub>f</sub> constants are the so-called complexation constants for both complexes respectively. As with all equilibrium
reactions, the larger the K<sub>f</sub>, the more the equilibrium leans to the right. We say that complexes with high
K<sub>f</sub> are strong complexes.
The K<sub>f</sub> values of many common complexes have been measured and the following table lists quite a few of them.
It follows mathematically that if one complex is stronger than another, the latter can be displaced by the former by adding ligands of the former. The
ligands of the weaker complex are then released. This phenomenon is called ligand exchange and can be quantified by the ligand exchange constant
K<sub>LN</sub>.
A commonly known and colourful ligand exchange reaction takes place when NaCl solution is added in sufficient quantity to a copper sulphate solution.
The latter is a blue solution which turns gradually green due on addition of the NaCl solution, to the ligand exchange reaction:
[Cu(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup>(aq, BLUE) + 4 Cl<sup>-</sup>(aq) < === >
[CuCl<sub>4</sub>]<sup>2-</sup>(aq, GREEN) + 6 H<sub>2</sub>O(l)
In Lewis acid base theory we’d say that chloride ions are ‘harder’ Lewis bases than water (strength of Lewis acids and bases is referred to as
being ‘hard’ or ‘soft’). The harder base thus displaces the softer one.
Aluminium’s amphoterism:
It’s well known that aluminium salts tend to be highly soluble in acid solutions as well as in strongly alkaline solutions. In between there’s a
pH zone where Al is insoluble (and precipitates as hydrated alumina).
Al(+3)’s solubility at both low and high pH is due to a ligand exchange reaction:
[Al(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq) + 4 OH<sup>-</sup>(aq) < ==== >
Al(OH)<sub>4</sub><sup>-</sup>(aq) + 6 H<sub>2</sub>O(l)
The relative concentrations (α) of various species of Al(+3) in the full pH interval are shown below:
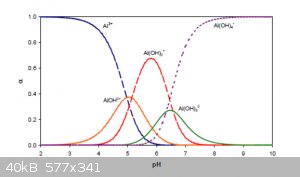
Note that by Al<sup>3+</sup> in this figure is really meant
[Al(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq) and that the intermediate species noted as
AlOH<sup>2+</sup> and Al(OH)<sub>2</sub><sup>+</sup> are the result of partial ligand exchanges.
The amphoterism of Zn, Pb, Sn, Cu, Sb, As and others can be explained in analogous ways.
[Edited on 26-11-2015 by blogfast25]
[Edited on 26-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ah yes. Of course.
Amphoteric are bisexual ions.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
It's really up to you where we go from here. I can suggest a few avenues to further explore if you like.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@aga:
First of all, LOL at "bisexual ions." Second of all, do you think you're ready for another round of organic chemistry, albeit a bit more advanced this
time around? You may have noticed that I've slowed down on the OC a bit these last couple of weeks. This was partly to give you time to catch up and
to prevent information overload. Between all of the QM lectures and extracurricular OC excursions, a lot of material has been covered since July. So
before we move on, do you have any questions regarding the concepts discussed thus far? Just to recap, here's what we've covered (not including the
additional OC contributions by blogfast):
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I have many questions, and feel that the answers to most of them are in the body of this thread.
If it would be OK, i'd like some time to re-, re- then re- read all of the Education you kind gents have posted here.
The day job tends to get in the way, and eats up far too much valuable Chemistry time.
Without being 100% sure if this is possible, perhaps QM and OC can be combined with some practical experiment at some point.
I got some pthalic anhydride today and there's phenol on it's merry way too.
Making phenolpthalein looks fairly straightforward, so perhaps if i make an attempt at the reaction mechanisms and the underlying QM, then discuss,
before doing the actual synthesis, perhaps that would be a good way to bring it all together into an actual physical manifestation of all these
wonderments.
Maybe that isn't a good reaction to illustrate the OC and QM, i do not know.
Suggestions welcome.
(OC reagents on hand are salicylic acid, sulfamic acid, toluene, methanol, super dry ethanol, inositol, choline bitartrate, acetone, ethyl acetate,
ascorbic acid, glacial acetic acid, plus all the usual IOC reagents as well as a ton of glassware, vac pump etc).
[Edited on 26-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I got some pthalic anhydride today and there's phenol on it's merry way too.
Making phenolpthalein looks fairly straightforward, so perhaps if i make an attempt at the reaction mechanisms and the underlying QM, then discuss,
before doing the actual synthesis, perhaps that would be a good way to bring it all together into an actual physical manifestation of all these
wonderments.
|
Have you got a particular synthesis route in mind?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
utoob is all. Nile Red.
Appologies if he stole anyone's writeup.
https://www.youtube.com/watch?v=kBo5UnNRodA
Edit:
There is No reason to use that particular synth other than it has pictures.
If there is a better, or more informative synth, then groovyness all round.
More Edit:
I already have phenolpthalein so actual Product at the end is largely irrelevant.
If there are Other experiments that would be more educational, then they would be more suitable.
[Edited on 26-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You would do well to transcribe the method here, in 'shorthand' so we can have a look at it. Maybe then we can suggest a QC based reaction mechanism,
if one is imaginable (I haven't watched the video yet).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. Gimme a few mins.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Transcript :-
2g phenol
1.5g pthalic anhydride
Add both to what a 25ml RBF.
Add 'a few drops' of 'concentrated' hydrochloric acid.
(i detest Both of those adjectives - why not just state conc and volume ?)
Heat, unstoppered, to 150 C for two hours.
Stirring is evident, although not stated.
Mixture goes red, clear red, nearly black.
Cool to RT.
Add 10 ml distilled water + 10ml dichloromethane (DCM).
continue stirring for an unspecified time.
Transfer to to separatory funnel.
Wash remainder from the RBF with 'some' DCM. into sep funnel.
Mix/shake/vent then allow to separate for an unspecified time.
Drain off lower layer (product).
Add 10ml more DCM to the residue in the sep funnel.
Shake 'n' vent, drain off lower layer again adding to earlier lower layer.
Transfer the combined product to another sep funnel.
Wash residues into the sep funnel with a 'small amount' of DCM.
Add 5ml 2[M] NaOH solution. (goes purple)
Add 10ml water.
Cap n shake n vent, let separate.
Drain off Lower layer and discard.
Drain Upper Product layer into a beaker. Wash sep funnel with water into same beaker.
Pour into 100ml 2[M] HCl (goes white and insoluble).
Vac filter. Vac dry.
1g of product.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks. With HCl when it states 'conc.' it always means 37 w% HCl (the maximum).
So it's a reaction between phthalic acid anhydride and phenol in acid conditions, the rest is basically work-up. Let's see what the Quantum Witches
have to say about that brew...
And now you've transcribed it, you won't have to 'cook from video'. The number of Lobster Thermidors I've buggered cooking that straight from the
teevee is frightening.
BTW: it calls for a few drops of conc. H2SO4, NOT HCl!
[Edited on 27-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Proposed Reaction Mechanism for the Synthesis of Phenolphthalein (addition/condensation of phenol to phthalic acid anhydride):
Conc. H2SO4 acts as a catalyst. It also pushes back the deprotonation of phenol (phenoxide ions might interfere here).
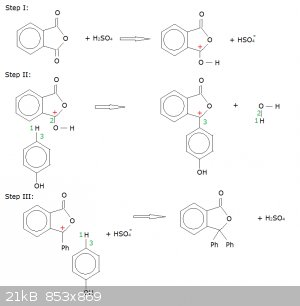
Step I: protonation of one of the carboxyl groups.
This creates a carbocation (marked with a red +) where the carboxyl group was.
Step II: first addition of a phenol.
Orbital 3 latches on to the carbocation, while proton 1 latches on to orbital 2, creating water as a leaving group. Note that these movements leaves
the carbocation in place, which explains why both phenols (Ph) are added to the same carbon atom and not one to each carboxyl group.
Step III: second addition of phenol and catalyst regeneration.
Orbital 3 latches onto the carbocation, proton 1 relocates to the HSO<sub>4</sub><sup>-</sup>, there by regenerating the
catalyst.
[Edited on 28-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Forgive what follows as noob misunderstanding if you would be so kind :
I can imagine ways that the reaction intermediates occur that would be different.
Drawing it may be difficult as the notion of the orbitals' shape around each element is hard to imagine, let alone draw.
[Edited on 27-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Forgive what follows as noob misunderstanding if you would be so kind :
I can imagine ways that the reaction intermediates occur that would be different.
Drawing it may be difficult as the notion of the orbitals' shape around each element is hard to imagine, let alone draw.
[Edited on 27-11-2015 by aga] |
Others may have a different opinion too, it's not chiselled in granite , this proposal. So go for it, Hombre!  Use whatever means to draw stuff: manually drawn and scanned stuff works too. Use whatever means to draw stuff: manually drawn and scanned stuff works too.
I was also looking at the Enthalpy of Reaction (Standard), using bond Enthalpies as explained above. Per mol of reaction product:
1 mol of C=O broken: costs + 749 kJ.
2 mol of H-Ph broken: costs 2 X + 431 kJ
2 mol of C-Ph formed: yields 2 X - 418 kJ
1 mol of H<sub>2</sub>O formed: yields - 286 kJ
Enthalpy per mol PP formed = +489 kJ
Even allowing for some uncertainty on the estimate (data points, non-STP temperature of execution, no Entropic term calculated) this suggests a
reaction that is not thermodynamically favourable.
But by carrying it out at 150 C, water is constantly removed from the reaction mix, driving the equilibrium:
PAA{s) + 2 Ph(l) ==== > PP(l) + H2O(g)
... to the right, in accordance with Le Chatelier's Principle. Endothermic equilibria are also driven right by high temperature.
[Edited on 28-11-2015 by blogfast25]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Thanks for that. For years I have looked at procedures which never specify other than stating 'concentrated'. For HCL I always assumed 37% as fairly
self explanatory, can you expand this to include other acids where that term is often used? Specifically these three: Nitric, Sulfuric, Acetic. Just
in general terms where one reads a procedure and the only term used is 'concentrated'. I have wondered about this many times. One acid where I think I
know what they mean is HF, where I always assumed they mean ~49%. Likewise I always assumed for Nitric they mean ~68%, but I am not really sure on
either this or HF.
By the way this is one of the better threads I have seen on SCM, thanks for putting in so much effort to teach this subject.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  |
Thanks for that. For years I have looked at procedures which never specify other than stating 'concentrated'. For HCL I always assumed 37% as fairly
self explanatory, can you expand this to include other acids where that term is often used? Specifically these three: Nitric, Sulfuric, Acetic. Just
in general terms where one reads a procedure and the only term used is 'concentrated'. I have wondered about this many times. One acid where I think I
know what they mean is HF, where I always assumed they mean ~49%.
By the way this is one of the better threads I have seen on SCM, thanks for putting in so much effort to teach this subject.
|
Hi IrC.
Thanks for the kudos, I appreciate it.
'Conc.' is a bit of a historical misnomer, I'm afraid. Practically it means the highest concentration that an acid can be obtained as with respect to
water as a solvent (or remainder or impurity).
Non-volatile mineral and stable acids like sulphuric, phosphoric and nitric acids can be worked up to about 100 w%. Even then different sub-grades
tend to exist on a case-by-case basis. But the rather volatile fatty acids (formic, acetic, propionic, butyric etc etc) can also be obtained as pure
substances. Glacial acetic acid means pure, anhydrous acetic acid but no one would refer to it is 'conc. acetic acid', though.
In the case of the hydrogen halides (gases in the pure form, except for HF (liquid)) 'conc.' means simply maximum solubility (at STP) which for HCl is
37 w% (approx. 12 M). The solubility limits of HF, HBr and HI you can of course find easily on teh Tinkerwebs.
Hope that helps...
[Edited on 28-11-2015 by blogfast25]
|
|
|
| Pages:
1
..
19
20
21
22
23
..
33 |