| Pages:
1
2
3
4 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nah. A half kilo chunk of 99%+ Zinc sounds better.
Well, up to the point where you have to hack off a 25g chunk, then the battery casings become much more appealing as they are thinner and easier to
cut.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
If you have the square lantern batteries (6V) they have 4 x 1.5V cells inside, when cleaning up dead batteries these always seemed to have the better
and thicker zinc in them. AAA batteries seem to have the worse (kind of make sense size wise).
The Duracell ones seemed to eat all the zinc if dead, the cheaper brand batteries that were dead had the most zinc left, just an assumption but maybe
the cheapo ones have cheaper electrolyte and therefore die before the zinc is used up?
Sorry nothing to back it up except taking apart one hell of alot of them lol
[Edited on 4-10-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Little_Ghost_again  |
The Duracell ones seemed to eat all the zinc if dead, the cheaper brand batteries that were dead had the most zinc left, just an assumption but maybe
the cheapo ones have cheaper electrolyte and therefore die before the zinc is used up?
|
Virgin or near-virgin would be best, definitely.
Taking apart with care doesn't even create much of a mess.
Recovering the Mn from the filler, now that IS messy!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Buying stuff from ebay seems equally messy.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Little_Ghost_again  |
The Duracell ones seemed to eat all the zinc if dead, the cheaper brand batteries that were dead had the most zinc left, just an assumption but maybe
the cheapo ones have cheaper electrolyte and therefore die before the zinc is used up?
|
Virgin or near-virgin would be best, definitely.
|
If you find a supply give me a shout  . .
Dont ask me, I only know enough to be dangerous
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Lemon Curd.
That's what the SnCl2 solution looks like after being boiled down a bit, complete witha slight frothiness on top.
Currently it is sitting in the lab fridge, hopefully forming non-lemon-curdy crystals.
The Zn solution is a complete failure - all i can get is Table Salt crystals out of it now.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Well, tossing the Zinc failure was probably a good idea.
The Tin Chloride went all Yellow and Thick.
Despite a few hot filtrations it remains yellow.
Leaving the light on in the shed overnight was a mistake.

The 'camoflage' moths come at this time every year.
I imagine it is the Male, who has mated already and just finds somewhere to die, which they always do.
This year they have very muted colours - usually they have bright red and orange colours.
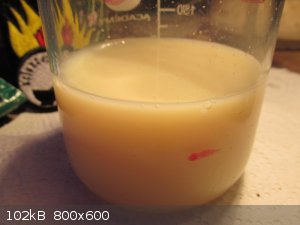
Diluting and filtering out the wildlife for the photo, THAT abomination is supposed to be Tin Chloride !
Closest colour match is the FeCl4<sup>-</sup> ion as per some website i cannot find today.
I think i will boil down some of my 20% HCl and see if it responds to the iron tests i have.
It shows no iron content in it's current concentration.
[Edited on 7-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Closest colour match is the FeCl4<sup>-</sup> ion as per some website i cannot find today.
I think i will boil down some of my 20% HCl and see if it responds to the iron tests i have.
It shows no iron content in it's current concentration.
|
Forget about iron, I'd put good money on there not being any.
Have you checked the solubility of the 'kurd'? If it doesn't dissolve to a clear solution you've got a problem of hydrolysis: basic chlorides having
been formed. If it does dissolve fully your product is good to go.
Highly soluble chlorides often don't form well-formed crystals.
[Edited on 8-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Soluble in water ?
If it is tin hydroxide/tin oxide, should i boil it down to a thick curd then bubble HCl gas through it for example, or is starting again with less
water the sane way to go ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Soluble in water ?
If it is tin hydroxide/tin oxide, should i boil it down to a thick curd then bubble HCl gas through it for example, or is starting again with less
water the sane way to go ? |
For once, do as you're told! 
Take a pinch of the kurdy stuff and see if it dissolves in a bit of water. Add a drop of acid to the water to make sure its pH < 7. Observe and
report. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yesbas.
No, the curdy stuff just goes into a milky yellow suspension/thin bannana milkshake on addition of water.
pH is around 1 already.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
No, the curdy stuff just goes into a milky yellow suspension/thin bannana milkshake on addition of water.
pH is around 1 already. |
Ok, that's hydrolysis for sure. Seems hard to avoid with SnCl2 dihydrate though I did make some that was fine. I also bought a batch for resale a
couple of years back and it too was 'bad'.
[Edited on 9-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So what is it after hydrolysis ?
Tin Hydroxide ?
More to the point, is it recoverable ?
I get the feeling that whacking some Cl2 into some elemental Tin would be easier.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The tin dioxide should be easy to dissolve by adding in conc. HCl, but then you will have a solution either just contaminated with hexachlorostannic
acid or mostly that, in short, tin in the 4+ oxidation state that needs to be reduced to 2+. If you're going to reduce this solution with tin metal,
keep the pH around -0.5 at all times and put suitable loose fitting plastic lid so that it doesn't get undue exposure to air or you might end up in
the same boat all over again (or even better, film plastic with a needle hole pricked in it as a vent) if you're leaving it for days open.
As for the colour, once you get the tin dioxide dissolved, simply stir your solution with a few grams of activated charcoal for several minutes, then
filter. It might help
Actually isolating stannous chloride crystals from you liquor after reduction might just prove too much of a pain. Use the solution as is... you want
it as a gold indicator right?
[Edited on 9-10-2015 by deltaH]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes, it is for a Gold indicator, so may be OK as-is.
However it HAS to become crystals, as that was the original objective.
I shit ye not : there WILL be SnCl2.2 H2O crystals around here some day soon.
Still got about 400g Sn left, and it has absolutely no choice in the matter.
[Edited on 9-10-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | The tin dioxide should be easy to dissolve by adding in conc. HCl, but then you will have a solution either just contaminated with hexachlorostannic
acid or mostly that, in short, tin in the 4+ oxidation state that needs to be reduced to 2+. If you're going to reduce this solution with tin metal,
keep the pH around -0.5 at all times and put suitable loose fitting plastic lid so that it doesn't get undue exposure to air or you might end up in
the same boat all over again (or even better, film plastic with a needle hole pricked in it as a vent) if you're leaving it for days open.
|
What he has is not primarily SnO2, the Sn(+2) will have partially oxidised to Sn(+4) by air but only partially. Real SnO2 is incredibly insoluble and
stubborn, it hardly responds to HCl at all.
I know this from personal experience: a bad batch of SnCl2 didn't even dissolve in 12 M HCl. Only on slowly and carefully adding cold H2O2 with
intermittent cooling was a clear solution of H<sub>2</sub>SnCl<sub>6</sub> obtained.
And yes, you can then reduce that back to SnCl2 with metallic Sn. That solution should be good enough for gold testing, without crystallising it.
[Edited on 9-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Peroxide going in the pot shortly.
Followed by a 400g block of Sn.
YeeHar !
There's Tin in them thar hills !
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
A squirt of H2O2 did the trick, even at just 3%.

Adding the cold lump of Tin metal took up most of the heat - no boiling or anything - the bar is a bit warm, and went a bit black.

Before LG2.373b says anything, No measurements were made.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Best to leave it stand overnight with the ingot in it, to ensure full reduction back to Sn(+2). The bottle it and put a few Sn chips in with the
solution. It will keep it 'always' as Sn(+2).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yesbas.
I'll try direct element combination over the weekend to see if i can get crystals, dust, an explosion - anything !
At some point soon there WILL be crystals.
There is no option - Giving Up at the first hurdle is just, well, Feeble.
[Edited on 9-10-2015 by aga]
[Edited on 9-10-2015 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by deltaH  | The tin dioxide should be easy to dissolve by adding in conc. HCl, but then you will have a solution either just contaminated with hexachlorostannic
acid or mostly that, in short, tin in the 4+ oxidation state that needs to be reduced to 2+. If you're going to reduce this solution with tin metal,
keep the pH around -0.5 at all times and put suitable loose fitting plastic lid so that it doesn't get undue exposure to air or you might end up in
the same boat all over again (or even better, film plastic with a needle hole pricked in it as a vent) if you're leaving it for days open.
|
What he has is not primarily SnO2, the Sn(+2) will have partially oxidised to Sn(+4) by air but only partially. Real SnO2 is incredibly insoluble and
stubborn, it hardly responds to HCl at all.
I know this from personal experience: a bad batch of SnCl2 didn't even dissolve in 12 M HCl. Only on slowly and carefully adding cold H2O2 with
intermittent cooling was a clear solution of H<sub>2</sub>SnCl<sub>6</sub> obtained.
And yes, you can then reduce that back to SnCl2 with metallic Sn. That solution should be good enough for gold testing, without crystallising it.
[Edited on 9-10-2015 by blogfast25] |
Ah ok, cool, I'm happy it all worked out for aga. I also have a rather large bar of supposedly very pure tin that I should convert at
some stage. I'm curious if this yellow curds is a universal behaviour.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  |
Ah ok, cool, I'm happy it all worked out for aga. I also have a rather large bar of supposedly very pure tin that I should convert at
some stage. I'm curious if this yellow curds is a universal behaviour. |
Funny that, I was thinking that too. I seem to recall having obtained some SnCl2 dihydrate crystals that were colourless.
There isn't much in the Sn<sup>2+</sup> [Kr] 4d<sup>10</sup>5s<sup>2</sup> electron configuration that would point
to colour.
[Edited on 10-10-2015 by blogfast25]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The 1851 translation of Gmelin mentions it, so it's nothing new. It seems clear from old refs (that googling stannous oxychloride brings up) that air
or a large amount of water is bad, and different bad things can happen.
Looks like it might be a good idea after all to follow the directions of dead chemists whose education was slightly less newfangled.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The directions given by some dead chemists will be followed on the next attempt.
Foofing up the synthesis like this teaches one a lot more than if it just worked perfectly every time.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by S.C. Wack  |
Looks like it might be a good idea after all to follow the directions of dead chemists whose education was slightly less newfangled.
|
Hear hear!
|
|
|
| Pages:
1
2
3
4 |