Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Reaction Mechanism Confirmation
Hey!
I've tried to propose a reaction mechanism between bismuth subsalicylate and hydrochloric acid. I've found no references to this online, so I've
basically worked it up from scratch. Something seems off though.
Could anyone please confirm?
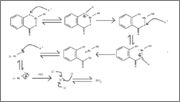
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Your electron-pushing arrows are going the wrong direction, for starters. They are going from protons, which have no electrons, to atoms. Reverse
that, and start from the beginning. Also, if this is for a reaction with hydrochloric acid (aqueous) and not dry HCl, water will take part in the
reaction and mechanism. Hopefully that should help set you straight.
[Edited on 17-10-2015 by Crowfjord]
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
D: Got the arrows half the arrows the other way around when I was editing in paint, you're right.
xD I actually missed that, thanks!
How is water involved in the reaction? I was thinking you could be formal and use hydronium ions as opposed to protons in the mechanism, but other
than that..?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sniffity  |
How is water involved in the reaction? I was thinking you could be formal and use hydronium ions as opposed to protons in the mechanism, but other
than that..? |
What is 'be formal' mean here?
No hydronium (oxonium) ions without water.
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Yeah, water's required to make hydronium ions alright, what I meant is for a protonation, the net result is the same whether you use a hydronium and
protonate or use a straight up proton. For the most part, I see straight up proton's used, but if you want to be "formal" I guess the right way to do
it is by using a hydronium ion, correct?
Is water involved in the reaction in any other way?
|
|
|