Difference between revisions of "Funnel-and-beaker trap"
| Line 3: | Line 3: | ||
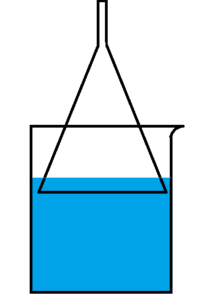
A '''funnel-and-beaker trap''' is a device for dissolving highly soluble gases in water. The trick with these gases, such as [[ammonia]] and [[hydrogen chloride]], is that they cannot be dissolved in water by simply bubbling them through it: the high solubility results in the water rushing back into the reaction vessel. The funnel-and-beaker trap is used to prevent that. | A '''funnel-and-beaker trap''' is a device for dissolving highly soluble gases in water. The trick with these gases, such as [[ammonia]] and [[hydrogen chloride]], is that they cannot be dissolved in water by simply bubbling them through it: the high solubility results in the water rushing back into the reaction vessel. The funnel-and-beaker trap is used to prevent that. | ||
| − | It works because of the short, wide part of the [[funnel]] allowing it to suck a large amount of water in. When the weight of the water becomes larger than the force of the atmospheric pressure forcing the water in, the water stops being sucked in. This apparatus has another mechanism of protection: if the above fails, the funnel sucks enough water for the water level in the [[beaker]] to drop below the level of the funnel rim, allowing air into the apparatus and forcibly stopping the | + | It works because of the short, wide part of the [[funnel]] allowing it to suck a large amount of water in. When the weight of the water becomes larger than the force of the atmospheric pressure forcing the water in, the water stops being sucked in. This apparatus has another mechanism of protection: if the above fails, the funnel sucks enough water for the water level in the [[beaker]] to drop below the level of the funnel rim, allowing air into the apparatus and forcibly stopping the [[suck back]]. |
| − | For the trap to work properly, the funnel and the beaker should be close in diameter. It is also advised to provide cooling for the beaker if a very concentrated solution is desired. | + | For the trap to work properly, the funnel and the beaker should be close in diameter. It is also advised to provide cooling for the beaker if a very concentrated solution is desired.<ref>https://www.youtube.com/watch?v=kqQSpRus-t0</ref> |
==References== | ==References== | ||
Latest revision as of 21:55, 26 December 2022
A funnel-and-beaker trap is a device for dissolving highly soluble gases in water. The trick with these gases, such as ammonia and hydrogen chloride, is that they cannot be dissolved in water by simply bubbling them through it: the high solubility results in the water rushing back into the reaction vessel. The funnel-and-beaker trap is used to prevent that.
It works because of the short, wide part of the funnel allowing it to suck a large amount of water in. When the weight of the water becomes larger than the force of the atmospheric pressure forcing the water in, the water stops being sucked in. This apparatus has another mechanism of protection: if the above fails, the funnel sucks enough water for the water level in the beaker to drop below the level of the funnel rim, allowing air into the apparatus and forcibly stopping the suck back.
For the trap to work properly, the funnel and the beaker should be close in diameter. It is also advised to provide cooling for the beaker if a very concentrated solution is desired.[1]