Difference between revisions of "Potassium chloride"
(Created page with "300px '''Potassium chloride''' is a salt with the formula KCl. It is a white crystalline solid. It is edible, commonly used as a salt sub...") |
|||
| (16 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[ | + | {{Chembox |
| − | '''Potassium chloride''' is a salt with the formula KCl. It is a white crystalline solid. It is edible, commonly used as a salt substitute, but it has a sharp, biting aftertaste (which is often described as metallic) that many people find unpleasant. | + | | Name = Potassium chloride |
| + | | Reference = | ||
| + | | IUPACName = Potassium chloride | ||
| + | | PIN = | ||
| + | | SystematicName = | ||
| + | | OtherNames = Muriate of potash<br>Sylvine<br>Sylvite | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Potassium_chloride.jpg | ||
| + | | ImageSize = 300 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White solid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 1420 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 1.984 g/cm<sup>3</sup> | ||
| + | | Formula = KCl | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 74.5513 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 770 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| + | | pKa = ~7 | ||
| + | | pKb = | ||
| + | | Solubility = 35.5 g/100 ml (20 °C) | ||
| + | | SolubleOther = Soluble in alkali, [[glycerol]]<br>Slightly soluble in [[alcohol]]<br>Insoluble in [[diethyl ether]], hydrocarbons | ||
| + | | Solvent = | ||
| + | | VaporPressure = ~0 mmHg | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = Octahedral | ||
| + | | CrystalStruct = Face centered cubic | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = −436 kJ/mol | ||
| + | | Entropy = 83 J·mol<sup>−1</sup>·K<sup>−1</sup> | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = Non-flammable | ||
| + | | ExploLimits = Non-explosive | ||
| + | | ExternalMSDS = [https://www.docdroid.net/zYI9Vnx/potassium-chloride-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = Non-flammable | ||
| + | | LD50 = 2,600 mg/kg (rat, oral) | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Lithium chloride]]<br>[[Sodium chloride]]<br>[[Rubidium chloride]]<br>[[Caesium chloride]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Potassium chloride''' is a salt with the formula '''KCl'''. It is a white crystalline solid. It is edible, commonly used as a salt substitute, but it has a sharp, biting aftertaste (which is often described as metallic) that many people find unpleasant. | ||
| − | [[ | + | It can be found in nature as the mineral ''sylvite'' or ''sylvine''. It is also found in the mineral ''sylvinite'', where it can be found with [[sodium chloride]] (halite). |
| − | == | + | |
| + | ==Properties== | ||
===Physical=== | ===Physical=== | ||
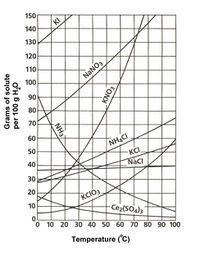
| − | Potassium chloride is a white crystalline solid with a cubic structure. It has a molar mass of 74.55. | + | Potassium chloride is a white crystalline solid with a cubic structure. It has a molar mass of 74.55. KCl's solubility in water is 28 g/100 mL at 0 ˚C and 56.7 g/mL at 100 ˚C<ref>CRC Handbook of Chemistry and Physics 66th edition</ref> |
| + | |||
| + | [[File:Sol_curve.jpg|thumb|200px|Solubility chart]] | ||
| + | |||
===Chemical=== | ===Chemical=== | ||
| + | Potassium chloride can be used as a source of chloride ions in reactions, although sodium chloride is more common. KCl is often used to make KClO<sub>3</sub> ([[potassium chlorate]]) using electrolysis. The high solubility of KCl at low temperature compared to KClO<sub>3</sub> makes it easy to separate the two compounds from a solution. | ||
| − | + | Although potassium is more [[electropositive]] than [[sodium]], KCl can be reduced to the metal by reaction with metallic sodium at 850 °C because the more volatile potassium can be removed by distillation (see [[Le Chatelier's principle]]): | |
| + | : KCl<sub>(l)</sub> + Na<sub>(l)</sub> ⇌ NaCl<sub>(l)</sub> + K<sub>(g)</sub> | ||
| + | |||
| + | Electrolysis of potassium chloride will give [[potassium hypochlorite]] at low temperatures, but if the reaction takes place at higher temperatures, [[potassium chlorate]] is produced. | ||
| + | |||
| + | :2 Cl<sup>−</sup> → Cl<sub>2</sub> + 2 e<sup>−</sup> (at the [[anode]]) | ||
| + | :2 H<sub>2</sub>O + 2 e<sup>−</sup> → H<sub>2</sub> + 2 HO<sup>−</sup> (at the [[cathode]]) | ||
| − | |||
==Availability== | ==Availability== | ||
| − | Potassium chloride can be easily obtained in relatively pure form at the grocery store as a salt substitute for people with low-sodium diets. This source is mixed in with [[potassium | + | [[File:Potassium_chloride_salt_substitute by Zts16.jpg|thumb|120px|Potassium chloride salt substitute]] |
| + | |||
| + | Potassium chloride can be easily obtained in relatively pure form at the grocery store as a salt substitute for people with low-sodium diets. This source is mixed in with [[potassium bitartrate]] to improve taste. Some salt substitutes tend to have merely a KCl addition, as still have lots of sodium chloride. Recrystallization is required to further purify the compound. | ||
| + | |||
| + | However, salt substitute is deliberately overpriced by the companies that make it; it is many times cheaper to obtain potassium chloride through other means, often through larger industrial quantities, which are usually purer anyway. This includes buying potassium chloride as fertilizer, sometimes referred to as muriate of potash. In some hardware stores, sodium-free water purification tablets made of 99% or higher potassium chloride can be purchased, usually in bags weighing about 40 lbs. These are by far the most economic method of purchasing potassium chloride. | ||
| + | |||
| + | Potassium chloride can also be bought cheaply in bulk as sodium-free chloride fertilizer. It can be identified after its NPK number 0-0-60, although some formulations may use 0-0-61. | ||
| + | |||
==Preparation== | ==Preparation== | ||
| − | Potassium chloride can be prepared from [[ | + | Potassium chloride can be prepared from [[potassium hydroxide]] and [[hydrochloric acid]], but this method is uneconomical in the lab due to the fact that KCl is usually easier to get than potassium hydroxide. [[Potassium carbonate]] or [[potassium bicarbonate]] can also be added to hydrochloric acid, but this requires care to control carbon dioxide outgassing. |
| + | |||
| + | Potassium chloride can also be obtained from saline water (seawater), through fractional recrystallization, though you will need a very large amount of seawater to obtain any useful amount of KCl. | ||
| + | |||
==Projects== | ==Projects== | ||
| − | *Make [[potassium chlorate]] | + | *Make [[potassium chlorate]] and [[potassium perchlorate]] |
| + | *Make potassium hydroxide and potassium hypochlorite | ||
| + | *Make potassium metal | ||
*Make colored flames | *Make colored flames | ||
| − | ==Safety== | + | *Growing crystals |
| + | *Salt bridge in electrochemistry | ||
| + | |||
| + | ==Handling== | ||
| + | ===Safety=== | ||
No safety measures are needed with potassium chloride. It is non-toxic unless directly consumed in very large quantities. | No safety measures are needed with potassium chloride. It is non-toxic unless directly consumed in very large quantities. | ||
| + | |||
| + | ===Storage=== | ||
| + | Potassium chloride should be stored in closed plastic or glass bottles. No special storage is required. | ||
| + | |||
| + | ===Disposal=== | ||
| + | Potassium chloride can be safely poured down the drain, soil or dumped in trash. | ||
| + | |||
==References== | ==References== | ||
<references /> | <references /> | ||
| + | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=28911 Is potassium chloride useful for any experiments?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=3196 Separating KCl and NaCl] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=25040 Potassium Chloride electrolysis] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Inorganic compounds]] | ||
| + | [[Category:Potassium compounds]] | ||
| + | [[Category:Chlorides]] | ||
| + | [[Category:Halides]] | ||
| + | [[Category:Neutral salts]] | ||
| + | [[Category:Minerals]] | ||
| + | [[Category:Chemicals for crystal growing]] | ||
| + | [[Category:Edible chemicals]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Materials available as food grade]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 14:47, 18 November 2023

| |
| Names | |
|---|---|
| IUPAC name
Potassium chloride
| |
| Other names
Muriate of potash
Sylvine Sylvite | |
| Properties | |
| KCl | |
| Molar mass | 74.5513 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.984 g/cm3 |
| Melting point | 770 °C (1,420 °F; 1,040 K) |
| Boiling point | 1,420 °C (2,590 °F; 1,690 K) |
| 35.5 g/100 ml (20 °C) | |
| Solubility | Soluble in alkali, glycerol Slightly soluble in alcohol Insoluble in diethyl ether, hydrocarbons |
| Vapor pressure | ~0 mmHg |
| Acidity (pKa) | ~7 |
| Thermochemistry | |
| Std molar
entropy (S |
83 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−436 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
2,600 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Lithium chloride Sodium chloride Rubidium chloride Caesium chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium chloride is a salt with the formula KCl. It is a white crystalline solid. It is edible, commonly used as a salt substitute, but it has a sharp, biting aftertaste (which is often described as metallic) that many people find unpleasant.
It can be found in nature as the mineral sylvite or sylvine. It is also found in the mineral sylvinite, where it can be found with sodium chloride (halite).
Contents
Properties
Physical
Potassium chloride is a white crystalline solid with a cubic structure. It has a molar mass of 74.55. KCl's solubility in water is 28 g/100 mL at 0 ˚C and 56.7 g/mL at 100 ˚C[1]
Chemical
Potassium chloride can be used as a source of chloride ions in reactions, although sodium chloride is more common. KCl is often used to make KClO3 (potassium chlorate) using electrolysis. The high solubility of KCl at low temperature compared to KClO3 makes it easy to separate the two compounds from a solution.
Although potassium is more electropositive than sodium, KCl can be reduced to the metal by reaction with metallic sodium at 850 °C because the more volatile potassium can be removed by distillation (see Le Chatelier's principle):
- KCl(l) + Na(l) ⇌ NaCl(l) + K(g)
Electrolysis of potassium chloride will give potassium hypochlorite at low temperatures, but if the reaction takes place at higher temperatures, potassium chlorate is produced.
Availability
Potassium chloride can be easily obtained in relatively pure form at the grocery store as a salt substitute for people with low-sodium diets. This source is mixed in with potassium bitartrate to improve taste. Some salt substitutes tend to have merely a KCl addition, as still have lots of sodium chloride. Recrystallization is required to further purify the compound.
However, salt substitute is deliberately overpriced by the companies that make it; it is many times cheaper to obtain potassium chloride through other means, often through larger industrial quantities, which are usually purer anyway. This includes buying potassium chloride as fertilizer, sometimes referred to as muriate of potash. In some hardware stores, sodium-free water purification tablets made of 99% or higher potassium chloride can be purchased, usually in bags weighing about 40 lbs. These are by far the most economic method of purchasing potassium chloride.
Potassium chloride can also be bought cheaply in bulk as sodium-free chloride fertilizer. It can be identified after its NPK number 0-0-60, although some formulations may use 0-0-61.
Preparation
Potassium chloride can be prepared from potassium hydroxide and hydrochloric acid, but this method is uneconomical in the lab due to the fact that KCl is usually easier to get than potassium hydroxide. Potassium carbonate or potassium bicarbonate can also be added to hydrochloric acid, but this requires care to control carbon dioxide outgassing.
Potassium chloride can also be obtained from saline water (seawater), through fractional recrystallization, though you will need a very large amount of seawater to obtain any useful amount of KCl.
Projects
- Make potassium chlorate and potassium perchlorate
- Make potassium hydroxide and potassium hypochlorite
- Make potassium metal
- Make colored flames
- Growing crystals
- Salt bridge in electrochemistry
Handling
Safety
No safety measures are needed with potassium chloride. It is non-toxic unless directly consumed in very large quantities.
Storage
Potassium chloride should be stored in closed plastic or glass bottles. No special storage is required.
Disposal
Potassium chloride can be safely poured down the drain, soil or dumped in trash.
References
- ↑ CRC Handbook of Chemistry and Physics 66th edition