| Pages:
1
..
51
52
53
54
55
..
104 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by luminouspath  | | I'm planning on concentrating some 12% H2O2 to 30% by removing water under vacuum at ~30c, is this as safe as I think it is? |
Assuming it's possible and you've got good control over final strength it should be.
I would give my glass ware a bit of a deep clean to avoid any oxidisables in there.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4326
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by 1.6180339  | | In areas with too much sulfur dioxide, could we[in a industrial process] convert it into sulfur trioxide, make it react with water to create sulfuric
acid and then make it react with sucrose to make carbon which could be used for industrial processes? |
There's far more cost-effective sources for carbon. Charring wood is much cheaper, and if you had to make it from sugar, it's easier to do it with
heat than with sulphuric acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
|
Threads Merged
29-2-2016 at 21:40 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by 1.6180339  | | In areas with too much sulfur dioxide, could we[in a industrial process] convert it into sulfur trioxide, make it react with water to create sulfuric
acid and then make it react with sucrose to make carbon which could be used for industrial processes? |
Not usefull except for desesperate people.
This is like killing a fly with an atomic bomb...way too much energy and money expenses for such a little result.
See differences between "efficacy", "efficiency", "effectuality", "effectiveness".
If you have that much SO2 better cath it by liquefaction or into NaOH solution (NaHSO3 and Na2SO3) and this would be more interesting as reducer.
The making of SO3 cost energy, the concentration of H2SO4 to a level able to dehydrate sucrose is another barrier.
The use of sucrose to make carbon is an energetical and financial crime.
The use of conc H2SO4 to dehydrate sucrose to C is another chemical crime.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Hello!
I need semipermeable membranes, the one I am talking about are these used to separate the electrodes in car batteries.
They are perfect for the experiments wih electrochemistry I want to start with, but I have no battery to wreck for them.
Can one buy these membranes somewhere separate, online or offline?
I guess that is possible but I don´t know how they are called, or where one would look after them.
It would be deeply appreciated if someone could help me out here.
[Edited on 3-3-2016 by karlos³]
[Edited on 3-3-2016 by karlos³]
|
|
|
Deathunter88
National Hazard
   
Posts: 518
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Potassium Chlorate -> Barium Chlorate
I have some potassium chlorate that I made through electrolysis. Since I'm not really into pyrotechnics I want to convert it into another more soluble
chlorate. At the top of my list are magnesium, calcium, strontium, and lithium chlorates. As an intermediate I plan to use the barium chlorate +
sulphuric acid route to make chloric acid, and then react that with a suitable carbonate. But here lies the problem, I cannot find a way to convert
potassium chlorate into barium chlorate. The 2 or 3 threads already on this forum about this issue all ended with a theoretical dead end so that is
why I chose to post this question in the short questions thread.
This conversion does not seem doable with a metathesis reaction since the only chlorate salts that is less soluble than potassium chlorate are caesium
and rubidium (both way too expensive to use). Perhaps a member here has some way?
Or maybe this is simply impossible and I will just toss my chlorate. 
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Maybe this could be done with an ion-exchange resin of some sort, loaded with barium. However, this would likely be expensive.
Why couldn't you just put barium chloride in a chlorate cell (or for that matter, the chloride salt of your choice)?
|
|
|
Deathunter88
National Hazard
   
Posts: 518
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Metacelsus  | Maybe this could be done with an ion-exchange resin of some sort, loaded with barium. However, this would likely be expensive.
Why couldn't you just put barium chloride in a chlorate cell (or for that matter, the chloride salt of your choice)? |
I guess I could just electrolyse some calcium chloride...
But since I already have the KClO3 I don't want to throw it away, and I hate having to clean up the huge hexavalent chromium mess my cell makes after
running which is why I was hoping I would never have to do electrolysis ever again.
|
|
|
Sulaiman
International Hazard
    
Posts: 3685
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
karlos³
I am a noob just starting to experiment with galvanic cells,
I think that for Lead-Acid cells it is more of a physical separator and to prevent dendrites shorting out the cell.
Almost any porous materials such as woven or wool fiberglass, plastic kitchen scourer etc...
In other situations such as the Daniel cell the membrane allows ions to squeeze through the pores
but prevents bulk mixing of electrolytes either side.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Sulaiman  | I am a noob just starting to experiment with galvanic cells,
I think that for Lead-Acid cells it is more of a physical separator and to prevent dendrites shorting out the cell.
Almost any porous materials such as woven or wool fiberglass, plastic kitchen scourer etc...
In other situations such as the Daniel cell the membrane allows ions to squeeze through the pores
but prevents bulk mixing of electrolytes either side. |
Thank you for your reply!
I am also just beginning with electrochemistry but I already know that a lot of alternatives for the cell divider can be used.
But in my case I have to use a different strenght of eletrolyte in each part of the cell.
These membranes look mostly attractive to me because they don´t have a high resistance so the solution does not heat up very much.
I could also take something from unglazed porcellain but then I have to cool my cell because of that high resistance.
I have to research if a daniel cell would be a suitable alternative for my experiment but it seems unlikely.
|
|
|
Maker
Harmless

Posts: 46
Registered: 1-11-2015
Member Is Offline
Mood: No Mood
|
|
What advantage does a RBF offer over a conical flask for distillation/reflux?
I'm considering a jointed conical flask or two for distillation as I don't have a heating mantle but there must be a reason why RBFs are used far more
often.
|
|
|
Sulaiman
International Hazard
    
Posts: 3685
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
For (partial) vacuum distillation an Erlenmeyer would have to be made of thicker glass than an rbf
especially the flat base.
Thick glass cannot support as large a temperature shock or difference as thin
http://www.kavalier.cz/en/section/32-simax-glass-mass.html
so maximum operating temperatures of an Erlenmeyer must be less than an rbf
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
I believe the main reason RBF's are preferred is because they are better suited to high temperatures. When an RBF reaches high temperatures and the
glass expands slightly, it expands outwards evenly because of its spherical shape. However, when the bottom of a erlenmeyer heats up and expands, it
cannot evenly expand outwards because it is flat, and if the heating is extreme enough or the glass low quality enough, it will often fracture.
That being said conical flasks are usually acceptable at low temperatures, but for higher temp operations I recommend looking into a sand, air, oil,
or salt bath if you do not have access to a heating mantle.
Edit: Looks like Sulaiman beat me to the punch!
[Edited on 3-15-2016 by Zephyr ]
|
|
|
Maker
Harmless

Posts: 46
Registered: 1-11-2015
Member Is Offline
Mood: No Mood
|
|
That makes sense.  Oil bath sounds like the way forward. Oil bath sounds like the way forward.
I've seen a couple of other types of flask at school too, one is pear shaped which is apparently to allow one to scrape thick/solid residues off the
walls and another shaped like an inverted pear (Like an RBF but with a pointed bottom). What are they both called and what is the latter one for?
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Hi all,
Quick question about rust. Just acquired a piece of equipment with rusty bolts and nuts... They are custom bolts and can't be replaced, so I removed
all of them and dipped them in rust remover solution (diluted phosphoric acid) which successfully removed all the rust... rinsed the nuts in hot water
to remove all traces of phosphoric acid, dried them thoroughly with hot air, and then dropped the nuts in a jar filled with WD40... a day later, i
patted dry all the hardware.
Before reinstalling the nuts, is there something I should do to prevent oxidation from coming back since they are now down to bare metal with no
protective coating on them? I would rather not paint them since the nuts and bolts will be screwed on/off quite often.
I remember a long time ago, I had a spray can lubricant called Moly-kote which protected quite well bare metal parts but on the down side, was a stain
hazard for fabrics...
Any suggestions for non-staining metal protection? Thanks!
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Here's an image of the parts in question, they're rack-mount brackets, which originally had a golden iridescent coating (cadmium?) but it had started
rusting at the edges and close to the threads. The phosphoric acid bath brought it down to the bare gray metal.

--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Maker
Harmless

Posts: 46
Registered: 1-11-2015
Member Is Offline
Mood: No Mood
|
|
I would put a bolt through them (To protect the threads) and give them a going over with some black rattlecan.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
In class, I have prepared a Nickel complex where I change 2 CH3COO ligands for 2 NO2 ligands. The procedure asks us to mix NaNO2 with NH4CH3COO and
then add it to the complexe. I don't understand why we don't just use directly NaNO2 instead of making NH4NO2 ??
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
j_sum1
Administrator
       
Posts: 6310
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
What can I do with Rosemary?
I have a large rosemary bush at my place. I like to keep it trimmed down to six foot but it is again reaching the eaves of my house and so needs 3-4
foot chopped off (yet again).
It tastes great by the way -- but obviously much more than can be used for culinary purposes.
I recall reading recently of some interesting compounds that might be extracted from the plant, but I cannot remember what they are. In the absence
of any better information I thought I'd begin with a steam distillation of the "essential oil". (I haven't done a steam distill before so it will be
a good exercise.)
Are there any good syths or extractions I can do from the oil or from the leaves directly?
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Why do the Chinese use this ubiquitous shape to display so many products?
http://www.alibaba.com/product-detail/Original-Taste-Pure-In...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by j_sum1  | I have a large rosemary bush at my place. I like to keep it trimmed down to six foot but it is again reaching the eaves of my house and so needs 3-4
foot chopped off (yet again).
It tastes great by the way -- but obviously much more than can be used for culinary purposes.
I recall reading recently of some interesting compounds that might be extracted from the plant, but I cannot remember what they are. In the absence
of any better information I thought I'd begin with a steam distillation of the "essential oil". (I haven't done a steam distill before so it will be
a good exercise.)
Are there any good syths or extractions I can do from the oil or from the leaves directly? |
Essential oils of the terpenic familly found in Rosemary:
Borneol
Cineol
Eucalyptol
Camphene
Pinene
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by alexleyenda  | | In class, I have prepared a Nickel complex where I change 2 CH3COO ligands for 2 NO2 ligands. The procedure asks us to mix NaNO2 with NH4CH3COO and
then add it to the complexe. I don't understand why we don't just use directly NaNO2 instead of making NH4NO2 ?? |
Probably to buffer the reaction pH. Ammonium acetate is a pH=7 buffer
Quote: Originally posted by j_sum1  | I recall reading recently of some interesting compounds that might be extracted from the plant, but I cannot remember what they are. In the absence
of any better information I thought I'd begin with a steam distillation of the "essential oil". (I haven't done a steam distill before so it will be
a good exercise.)
Are there any good syths or extractions I can do from the oil or from the leaves directly? |
Possibly not a great paper, but the composition of most rosemary essential oil is probably pretty similar. Additionally, rosemary leaf is about
1.5-2.5% essential oil when fresh, so a very large amount of material must be steam distilled to produce a reasonable yield. It's certainly better
than some other materials used for essential oils, though.
http://idosi.org/aejaes/jaes5(1)/13.pdf
You'll note that the main components are alpha-pinene, camphene, 1,8-cineole, and camphor. It's a pretty complex mixture without a dominant component
and the components would all be much more easily available from other essential oils. Camphor can just be purchased pure, pinene makes up the majority
of turpentine, eucalyptus globulus oil is almost all 1,8-cineole, and nutmeg essential oil is mostly camphene.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
damned ferlilizer I picked up the other days is giving me fits--the prills have a green dye on them and if I boil a Urea solution do I risk getting a
bunch of biuret contamination?
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
This thread could have been easier to find...now here's the dumbass question: when would I resort to "computational chemistry?" Is it a biotech thing?
Do any of you use it and what sorts of problems can you address with it?
[Edited on 30-3-2016 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
does this compound exist?
no matches for pubchem and chemspider.
any help would be appreciated.
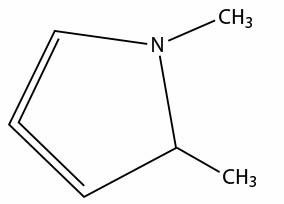
reality is an illusion 
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
no i doubt it, the doubly bonded carbon ( called a cumulated diene or allenes) is sp2 so would be linear and hence there would be significant ring
strain and the bond to N and C being stretched would make it very unstable. any one else want to add?
[Edited on 3-4-2016 by HeYBrO]
|
|
|
| Pages:
1
..
51
52
53
54
55
..
104 |