Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
tin salts thread
I decided to start a tin thread as there may be others who are interested this metal or its salts, or like me are frustrated by its vagaries and
perversities.
I have some of the mineral cassiterite. This mineral is virtually the world's only source of tin. The tin is in the form of high density (sp gr =
6.9) dark brown SnO2 nuggets in a matrix of silica, with some iron present. I have picked out some nuggets and ground them to a <90 micron
particle size powder for testing.
My first attempts were to dissolve the powder in acids including aqua regia. No dissolution.
Then I fused some powder with Na2CO3 in a muffle furnace. This dissolved it but assuming it was then Na2SnO3 (sodium stannate) I didn't know where to
go from there.
Then I tried to reduce it to tin with charcoal and Na2CO3 as flux in a muffle furnace at about 1000C. No go here either.
I have tried reducing it with a blowpipe on a bed of charcoal with added charcoal/Na2CO3 flux. This also didn't work probably because I couldn't get
a decent reducing flame cone onto the sample.
I have also tried fusing with sulfur in an attempt to produce sodium thiostannate, Na2SnS3. This apparently failed also.
The only test that has worked is a reduction with hydrogen bubbles generated with a piece of zinc laying next to a nugget of cassiterite in dilute
HCl. And here I just get many very tiny, shiny points of tin formed right on the nugget.
About the only thing I have not tried is to attempt a reduction in my tube furnace at 1200C.
Has anyone else been down this road before? Any suggestions for getting this cassiterite into a form of tin that can be qualitatively tested would
be appreciated.
[Edited on 5-4-2009 by Magpie]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Funny you bring this post up Magpie I was just thinking about trying to make tin salts a few hours ago. I was trying my damnest to figure out what if
anything around my house had tin to use but I came up with nothing.
My main goal was to produce the oxide though because I use it for polishing rocks. I haven't found a damn thing that works better as a finish polish
then tin oxide and its quick in a tumbler.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
sakshaug007
Hazard to Self
 
Posts: 94
Registered: 11-3-2009
Location: USA-Pacific Northwest
Member Is Offline
Mood: No Mood
|
|
You can try to reduce the cassiterite using the FFC Cambridge process if you form them into pellets and use them as the cathode in molten CuCl2. This
way it would only require ~500°C (to keep CuCl2 molten). If you try this let me know how it goes.
Hope this is helpful.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Purified SnO2 is available from pottery supply houses. Cassiterite contains copper and iron sulfides, as well as arsenates and tungstates of those
metals. An oxidising air roast is first needed, sulfur and arsenic oxides vaporising off. After that a strong magnetic field is used to separate out
some of the other oxides. Finally the oxide is mixed well with carbon and heated in a reverberatory or blast furnace to produce crude metallic tin
containing some copper and other metals.
Heating SnO2 with concentrated sulfuric acid will dissolve it, when the solution is slowly diluted (don't let it get very hot) a hydrated oxide
precipitates; a portion of this will dissolve in strong HCl or aqueous alkalies.
Heating with quite concentrated alkali will form stannates, as does the fusion with Na2CO3 you did, but the reaction is slow.
Passing CO2 into an aqueous solution of a stannate precipitates "alpha-metastannic acid", this will dissolve in hydrochloric acid.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Relevant: http://books.google.com/books?id=kuZ8fvczfZoC&pg=PA216
The amount of cyanide used there is excessive compared to the procedure given by Biltz.
[Edited on 5-4-2009 by S.C. Wack]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
How about that. Sn has been on my mind too. I was thinking of using SnCl2 to make an organometallic agent.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Cyanide is rather dear for something as mundane as preparing metallic tin, I think for most readers it would be easier and cheaper to buy tin metal.
Starting with ceramics grade tin oxide avoids the concentration workup needed for lower grade ores, as well as the oxidative roasting or other such
treatment.
The process in that book is for analysis, where accurate measuring of the amount of tin in an ore sample is the goal, rather than the normal
extractive processes used in the production of tin.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
If tin wouldn't dissolve when used as anode it would be a perfect one. But surely it isn't ... ; thats also a quite easy way to get towards any thin
compound, because it's easy to cast it, eg. to plates ..., and then to use it as anode.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Tin compounds are very usefull indeed.
I have recently been using acidic stannous chloride to reduce benzene diazonium chloride to phenyl hydrazine HCl, and i used alkaline stannous
chloride (sodium stannate) to reduce BDC to benzene.
Im sure there are lots of other uses out there...
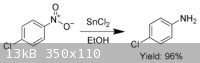
EDIT- Just found another use, can reduce aromatic nitro compounds to amines, with high (96%) yield.
[Edited on 5-4-2009 by Picric-A]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The nuggets of cassiterite that I have ground to a powder looked quite free from gangue. I would be surprised if they were not at least 90% SnO2.
But it will be easy enough to roast some powder in air and determine any weight loss.
The assay method using KCN looks interesting. I would try this right away if I had the KCN. I may have to make some. I wonder if cyanide is so much
better than charcoal as a reductant. This whole subject of reducing metal oxides with reductants like hydrogen, CO, CN-, etc, is very interesting and
I wish I knew more about it. My only real experience at doing this is in reducing PbO to Pb in the fire assay of gold ore. Here it seems that the
reduction is quite facile and almost any carbonaceous material can be used. For example, I used wheat flour.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
When i need tin i buy lead solder sticks of which are a 1:1 Pb:Sn alloy (well i assume this based on the fact that it is stamped on the stick). These
melt conveniently in a stainless teapot (minimise vapours and pourable) on a hotplate and the molten mix is poured as finely as possible into a bucket
of hot water. As you proceed the water slowly reaches 100C after which more hot water needs to be added to keep it betwen 74-100C.
When complete the water is poured off and the grains strained and divided. The shiny tin and the dull lead are readily separable by visual
inspection.
I then do it again on the tin, usually i get some.
I don't know how much lead ends up in my tin but its melting point is essentially that of the element, not that this is evidence of much. I have only
ever used tin in a reducing HCl procedure for which some lead impurity would not matter and as a filler for timber furniture made from acacia
hardwood's whose char point is beyond that of tins mp.
[Edited on 6-4-2009 by Panache]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The cyanide reduction has the advantage the the reducing agent is a liquid, wetting the ore; at the same time it and the oxidation products act as a
flux. Charcoal on the other hand is a solid and so you're dealing with solid-solid points of contact. Using charcoal along with a heavy oil or tar,
forming the ore-reducing agent mixture into chunks, might give better results.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I see your point about solid-solid reactions. Perhaps that is why my reduction with hydrogen was successful if only on a very small scale. One
reference said that the reduction with charcoal must be done at 1200C. This would melt the SnO2 thereby giving a solid-liquid reaction. At 1000C the
PbO in my gold assay would have been molten also.
Another possible approach would be to convert the SnO2 to sodium stannate, Na2SnO3, via fusion with Na2CO3. Then convert this to alpha-metastannic
acid, H2SnO3, using HCl. Further treatment with con HCl should yield SnCl6--. Reacting this with aluminum wire should reduce it to SnCl4--. In this
form it can be qualitatively tested with HgCl2 and verified as tin. Does this sound reasonable?
[Edited on 6-4-2009 by Magpie]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
I must ask do people here do their solid phase experimentation on pelletised and intimately ground/milled solids, as it can make an enormous
difference. I would assume standard practice is pelletised. If success via a published procedure is elusive it may be as simple as buying a 10tonne
workshop press. Of course if one is relying on the atmosphere to provide a reagent, pelletising would be counterproductive.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I usually grind up the reactants to a powder with mortar & pestle. But I'm not sure that any significant reaction takes place until at least one
of the reactants fuses.
But if you want a few small pellets there is really no need to buy a 10-ton press:
http://www.sciencemadness.org/talk/viewthread.php?tid=4761#p...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
| Quote: |
Another possible approach would be to convert the SnO2 to sodium stannate, Na2SnO3, via fusion with Na2CO3. Then convert this to alpha-metastannic
acid, H2SnO3, using HCl. Further treatment with con HCl should yield SnCl6--. Reacting this with aluminum wire should reduce it to SnCl4--. In this
form it can be qualitatively tested with HgCl2 and verified as tin. Does this sound reasonable?
|
Using my <90 micron "select" cassiterite I followed the above plan with the exception that I used NaOH instead of Na2CO3 for the fusion. I mixed a
stoichiometric amount of NaOH with the cassiterite and heated it as hot as possible in a nickel crucible using a regular Bunsen (tirrill) burner and
propane. It never really formed a liquid that I could see, just an ash-like product. I extracted this with a bit of water and then separated it to a
clear liquid with a centrifuge.
When 6M HCl was added drop by drop it first went cloudy white, presumably having formed alpha-metastannic acid. As the HCl addition was continued it
cleared up, presumably as SnCl6--. When this was reduced by hydrogen using a piece of aluminum wire it seemed that a flake of metallic tin was also
produced. The reduced liquid gave a positive test for tin via a drop of HgCl2.
It's likely my above estimation of the chemistry is an oversimplification, as the chemistry of tin is complex.
I'm going to attempt to reduce some of the powder to metallic tin using hydrogen. This hydrogen generation really is so easy and convenitent when
using a dilute acid and a tiny piece of aluminum or zinc.
Also, I still want to do a carbon reduction at 1200C.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
If you have tin metal, tin(IV) halides are easily synthesized. Tin metal reacts with iodine in a refluxing dichloromethane to produce tin (IV) iodide,
a dense orange colored compound. Tin (IV) bromide can be produced by slowly dripping dry bromine on to tin in a round bottomed flask. The tin reacts
with the bromine and gives quite the show of sparks. The product is then distilled and soon soldifies in the collection flask. The product fumes in
air. Tin (IV) chloride can be produced by heating tin in an atmosphere of dry chlorine and distilling the liquid produced. Again the show of sparks is
evident. Tin (IV) chloride pentahydrate can be produced by adding the stochiometric amount of water to the anhydrous chloride carefully. This compound
reacts with ammonium chloride to form ammonium hexachlorostannate. I have conducted these procedures and they work well. Tin (IV) compounds are useful
for making organotin compounds.
Amateur NMR spectroscopist
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
easy source of tin(II) chloride is lead free solder dissolved in hot hydrochloric acid. Ceramic crock pot works great. The copper, antimony,
whatever, does not seem to dissolve unless an oxidant is provided. Resulting solution, filtered through glass frit is very easy to electrolyze to
yield pure tin.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: |
[quote=151060&tid=12026&author=benzylchloride1]If you have tin metal, tin(IV) halides are easily synthesized............... Tin (IV) chloride
can be produced by heating tin in an atmosphere of dry chlorine and distilling the liquid produced. Again the show of sparks is evident. Tin (IV)
chloride pentahydrate can be produced by adding the stochiometric amount of water to the anhydrous chloride carefully. This compound reacts with
ammonium chloride to form ammonium hexachlorostannate. I have conducted these procedures and they work well. Tin (IV) compounds are useful for making
organotin compounds.
|
@BenzalChloride1
When you say 'adding the stochiometric amount of water to the anhydrous chloride carefully. ' (making Tin(IV) Chloride Pentahydrate) do you mean add
the water very slowly.
How slow? Do you use stirring? What temperature did you use (approx.)?
Have you actually carried out the procedure of converting the anhydrous Tin (IV) Chloride (liquid) into the hydrated (solid) form.
I thought Tin Oxide would form as soon as water was added.
TIA,
Dann2
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Hydrolosis of Tin Chlorides only occurs in either hot water or an exess of water.
You can safly hydrate anhydrous Tin (IV) Chloride by slowly adding water, drop by drop to the chloride, which is being cooled by ice.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thannks for that Piric-A.
A guy I communicated with some time ago took this from some publication.
_____________________
SnCl<small>4</small> (anhydrous) can be manufactured by applying Chlorine gas to Tin metal. The formed SnCl<small>4</small>
(liquid) should be allowed to drip onto the Tin metal in the apparatus. The reaction is exothermic. The Tin metal should be OK if cut into small
pieces or powdered.<br>
Anhydrous SnCl<small>4</small> will dissolve in cold water ( warm/hot will decompose it).<br>
The SnCl<small>4</small> should be added to a minimum amount of water and this solution saturated with Chlorine gas. The solution is
then evaporated and condensed. When this solution is cooled, deposits of SnCl<small>4</small>:5H<small>2</small>0 will form.
_______________________________________
I don't know what they mean when they say 'the solution shoud be evaporated and condensed'.
Surely this would leave the SnCl4 behind.
Anyhow some more info on Tin Chlorides attached.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thanks for that Picric-A,
Some snippets on Tin Chlorides attached.
I posted this yesterday and it fell out the bottom of Sci Madness. Must have been a server hicup.
Dann2
Attachment: stannic.pdf (242kB)
This file has been downloaded 703 times
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I've made crude tin metal by heating a mixture of pottery grade SnO2 and ground BBQ charcoal in a charcoal-fired mini furnace. Interesting to watch as
'bubbles' of CO (or CO2, not sure) escape from the powder and shiny pearls of tin metal start to form which then sink to the bottom and form a puddle
of molten, crude tin.
There is absolutely no reason why this wouldn't work with crude, micronised cassiterite. You do need red heat or higher. This is how Bronze Age
chemists did it. And what was good enough for them should be good enough for us...
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Cassiterite, SnO2, is not the only mineral source of tin. It also is found in some sulfide ores, as mixed Sn sulfides also containing varying amounts
of Cu, Zn, Cd, Pb, As, Sb, Bi, and Ge, e.g. redruthite (found in Cornwall, a mixed Cu and Sn sulfide) and germanite (a mixed Ge sulfide also
containing Cu and Sn, found in Namibia, formerly German Southwest Africa).
|
|
|