WannaBeDrD
Harmless

Posts: 10
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Separate CaCl2 from MgCl2
I have some ice melt that is a mixture of CaCl2 and MgCl2. I'd like to separate the two compounds, but I'm not quite sure how to do so since their
soluability characteristics are very similar and my knowledge of chemistry is fairly limited.
Any suggestions?
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I don't know whether it counts as separating them per se, but if you add Ca(OH)2 you get the reaction
MgCl2 + Ca(OH)2 -> Mg(OH)2 + CaCl2
(with the Mg(OH)2 as precipitate)
So, add an excess of Ca(OH)2 to an aqueous solution of the ice melt and all the magnesium ends up in the precipitate.
Not sure whether calcium carbonate would do the same trick but it seems like it should.
[Edited on 6-1-2011 by bbartlog]
|
|
|
WannaBeDrD
Harmless

Posts: 10
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Cool, thanks. That's what I was thinking would happen.
The reaction leaves me with a precipitate of Mg(OH)2. So to get that back to MgCl2, I would filter off the precipitate and react it with HCL, right?
But how much HCL to use...does it matter?
Ultimately, I want to end up with MgCl2 crystals.
And is this what the equation would look like:
Mg(OH)2 + HCL -> MgCl2 + 2H2O
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
I would suggest that you leave an excess of magnesium hydroxide, don't dissolve it all with the HCl, so that the HCl is completely reacted with the
hydroxide. Leaving a bit of the precipitate will ensure that your solution is nearly pure magnesium chloride with very little HCl left in it. You can
filter out the solution then to separate the remaining magnesium hydroxide precipitate.
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Ah, so the MgCl2 is your target. In that case my suggestion would not be the best, since the precipitate would contain Ca(OH)2 if that was used in
excess... and in that case adding HCl would just give you back your original problem. Even using less Ca(OH)2 than called for by theory might leave
you with the question of whether all of this insoluble compound had really reacted, or whether some of your precipitate was Mg(OH2) on the outside
covering some leftover Ca(OH)2 on the inside. If the MgCl2 is a relatively minor constituent then I guess this might still be the way to go, though.
If you know how much MgCl2 is in your ice melt, another thing you could do (given that it's the MgCl2 you want) is to add an appropriately sized
solution of epsom salt (MgSO4) to a solution of the ice melt. This would result in
CaCl2 + MgSO4 -> CaSO4 + MgCl2
...i.e. the Ca would mostly precipitate as CaSO4 and you'd end up with even more of your desired product in the solution. Assuming you get the
stoichiometry right you'd end up with a solution of almost all MgCl2, which you could then boil down for crystals. However CaSO4 is not entirely
insoluble, so there might be some minor contamination.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WannaBeDrD  | I have some ice melt that is a mixture of CaCl2 and MgCl2. I'd like to separate the two compounds, but I'm not quite sure how to do so since their
soluability characteristics are very similar and my knowledge of chemistry is fairly limited.
|
Curious do be I... this the stuff you put on you sidewalk to melt
snow?
This should keep you supplied for a day or two.
http://tinyurl.com/239sghk
|
|
|
WannaBeDrD
Harmless

Posts: 10
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Extract MgCl2 from Ice Melt with NaCl and MgCl2
@Wizard:
Yes this is the sidewalk stuff...different brand and MUCH smaller quantity. 
Right.
So...I'm very sorry to say that after checking the product label again to see if the percentages were listed, I realized I grabbed the wrong
container. I ended up getting the mixture NaCl, MgCl2 mixture, not the stuff with CaCl2. Sorry for the misinformation.
Still need to extract the MgCl2, though.
Would the following work, or is there a better way:
1) dissolve in HCL
2) filter out the undissolved NaCl (CRC says it's insoluble in HCL)
3) recrystallize the MgCl2
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
It probably has very little MgCl2 in it. Why not get a bunch of Mg fire starters and toss it in HCl? That would be way easier and you can set the
hydrogen on fire.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
WannaBeDrD
Harmless

Posts: 10
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
For that matter Mr. Crow, I could just buy some MgCl2 at the local pet shop. It's used in marine aquariums.
On the other hand, I needed the ice melt anyway (for it's intended purpose) and thought that it'd be fun do the extract for my other purpose.
Fire is good though...I'll keep that in mind for a summer-time project.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WannaBeDrD  | ...I ended up getting the mixture NaCl, MgCl2 mixture, not the stuff with CaCl2. Sorry for the misinformation.
Still need to extract the MgCl2, though.
... |
NaCl has a low solubility in organic solvents, even the smaller alcohols. MgCl2 (anhydrous or hydrated) has moderate solubility in many low weight
oxygen-containing solvents:
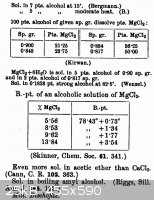
(from A dictionary of chemical solubilities Arthur Messenger Comey and available at Internet Archive or Google Books)
Thus you should be able to extract the MgCl2 using 95% EtOH (denatured but then distilled), MeOH, or 95-99 percent isopropanol. Might need to add a
few drops of hydrochloric acid to the alcohol to counteract hydrolysis of the MgCl2.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WannaBeDrD  | @Wizard:
Yes this is the sidewalk stuff...different brand and MUCH smaller quantity. 
Right.
So...I'm very sorry to say that after checking the product label again to see if the percentages were listed, I realized I grabbed the wrong
container. I ended up getting the mixture NaCl, MgCl2 mixture, not the stuff with CaCl2. Sorry for the misinformation.
Still need to extract the MgCl2, though. |
As inexpensive as calcium chloride is .... no one manufactures it
all that is found on the market is a waste product from the
manufacture off.... runs off the the shelves ... waste liquor
from the ammonia-soda process (Solvay). In the form of
3 : 1 calcium - magnesium chloride.
Book sez. MgCl is removed by treating w/ calcium oxide (lime),
which converts the chloride into the hydroxide. The Mg hydroxide
precips. and is removed by settling and decantation. (Followed
by details.)
CaCl goes for less than $20 for a 50lb bag around here. (95 CaCl)
the flake is a little less expensive then the prilled.
Interesting chemical waste...
Magnesium Waste
Hazardous waste finds use as low-cost fertilizer
Abstracted from: Chemical & Engineering News December 24, 1984
Phoenix Resource Recovery (PRR). PRR was created that year [1977] to
reprocess magnesium rich smelter slag from Northwest Alloys, Addy, Wash.,
a subsidiary of Aluminum Co. of America.
In the refining process, fluxing salts—potassium chloride and magnesium
chloride—are added to purify the magnesium metal and prevent it from
oxidizing. When the pour is made, some metal is left behind along with spent
fluxing salts. That combination is poured into molds, and the resulting sludge
bars, containing 18% magnesium, are trucked to PRR for recovery of the
metal, the company's principal business.
The recovery process is simple: The bars are crushed and the crushed
material is passed through screens. The malleable metal does not break
down and is captured on the screens. The material that does pass through
the screens consists of the fluxing salt residue and some fine magnesium
metal that cannot be recovered economically. PRR stockpiled the waste at its
plant site, and by the summer of 1983 the pile had grown to about 50,000
tons.
McLucas admits that the pile was unsightly and possessed some pretty
unpleasant properties. The fresh material contains 2% magnesium nitrides
which, upon contact with the atmosphere, hydrate to produce ammonia and
magnesium hydroxide. During temperature inversions in the valley, local
residents became rather aware of the ammonia emissions. Additionally, the
reaction is exothermic and occasionally generated sufficient heat to ignite the
fine magnesium metal in the mixture, which proceded what she calls
"spectacular fires."
|
|
|
WannaBeDrD
Harmless

Posts: 10
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Thanks very much for the reference material and information. I've added the book on solubilities to my library.
I think I'll try this out tomorrow using isopropanol since that's what I have on hand at the moment.
When you say "hydrolysis of the MgCl2", that would produce magnesium hydroxide, right? So the HCL prevents that by adding some extra Chlorine ions?
And how would I know whether I have MgCl2 or Mg(OH)2...testing the pH of the liquid?
|
|
|
DistractionGrating
Hazard to Self
 
Posts: 68
Registered: 3-4-2014
Member Is Offline
Mood: Precipitated
|
|
Let's assume that a particular bag of ice melt was largely MgCl2 contaminated with an unknown amount of LiCl, and the goal was to purify the MgCl2,
and specifically, to remove the LiCl? Any suggestions how to accomplish this?
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
This is a very old thread. Better to create a new thread.
The 2 basic methods :
-use a difference in solubility to crystallize one of them out of solution
-add some anion to precipitate one of the cathions.
1)
MgCl2 (anhydrous), in water
54.3 g/100 mL (20 °C)
LiCl, in water
84.25 g/100 mL (25 °C)
So it looks like you can crystallize LiCl first.
You could even add some anion and to crystallize.
2) MgCO3 is very insoluble so add a carbonate such as Na2CO3 or LiCO3 if you have it.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by vmelkon  | This is a very old thread. Better to create a new thread.
The 2 basic methods :
-use a difference in solubility to crystallize one of them out of solution
-add some anion to precipitate one of the cathions.
1)
MgCl2 (anhydrous), in water
54.3 g/100 mL (20 °C)
LiCl, in water
84.25 g/100 mL (25 °C)
So it looks like you can crystallize LiCl first.
You could even add some anion and to crystallize.
2) MgCO3 is very insoluble so add a carbonate such as Na2CO3 or LiCO3 if you have it. |
I'll take your solubility data at face value and they indicate that LiCl has slightly higher solubility of the two. If anything this means that if the
LiCl is a minority component then much of the MgCl2 could be crystallised (by boiling in), leaving most or all of the LiCl in solution together with
the rest of the MgCl2. But complete separation by crystallisation would require tedious fractionated crystallisation.
Li2CO3 is only sparingly soluble in water. Separation based on carbonates solubility would be far from ideal.
[Edited on 4-12-2014 by blogfast25]
|
|
|
DistractionGrating
Hazard to Self
 
Posts: 68
Registered: 3-4-2014
Member Is Offline
Mood: Precipitated
|
|
So, then it sounds like we're back to adding NaOH or Ca(OH)2, precipitating out Mg(OH)2, leaving the more soluble LiOH and NaCl or CaCl2 in solution,
then filter out the Mg(OH)2 and add HCl to yield MgCl2 and H2O.
|
|
|