palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Two-step preparation of biodiesel from waste soap
Hello people !
here another episode of the waste to resource experiment list: today we see the preparation of biodiesel from waste soap bars.
The procedure is a two-step synthesis, where the first was make fatty acids from expired soap, that has been already discussed on this forum before.
The second, which I am going to describe today is the esterification of such fatty acids with ethanol. The reaction we are going to perform is a
simple Fischer acid-catalyzed esterification.
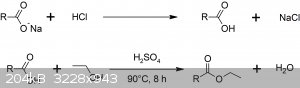
Procedure
216 g of fatty acids are suspended in 100 ml of denatured 90 deg ethanol are added; the suspension stirred for a while, then poured into 500 ml round
bottomed flask. The acids rinsed with additional 50 ml of ethanol, then this poured into reaction flask too. In total, 200 g circa of fatty acids are
employed as substrate; 2 ml of concentrated sulfuric acid are injected with a pipette. The flask is place in oil bath, and gently refluxed under
stirring for a total of 8 h. Temperature of the bath is kept around 90 Celsius. The acids take many hours to dissolve, and never got completely.
At the end of reaction, the flask is removed from bath, let cool, then filtered and aqueous layer extracted in separatory funnel. The organic layer is
then extracted with 1 x 50 ml each of sodium bicarbonate solution, water and brine. A terrible emulsion formed that require repeated water and brine
washings, and warming on hotplate to break, and at the end I did not succeeded completely, and ended with an opalescent yellow liquid.
This product is heated gradually to 120 Celsius under stirring on hotplate, in order to remove any volatiles, water and ethanol. It darkens to
orange-brown. Once cool, a precipitate forms. This suspension is vacuum filtered and 51 g / 56 ml of orange-brown viscous oily liquid is obtained,
along with 40 g circa of a waxy solid.
d = 0.91 g/ml

Discussion
The yield is terrible, because theoretically I had to get more than 200 g of product. The whole procedure is not convenient and anti-economical. But,
considering the huge, enormous losses due to the emulsion, I feel lucky I succeeded to finish the experiment, and got a product.
The reference I have, tells to have high yield in esterification of fatty acids with ethanol, a solvent as toluene must be used, otherwise a double 24
h esterification is needed. Since, I have not toluene nor the will to do such long esterification, I am happy with that; it has been better than throw
the waste soap bars to the garbage.
If anyone want to repeat, my suggestions are to separate out the aqueous layer, then evaporate off all volatiles, and after extract with bicarbonate,
brine and so on.
About the solid products, I did some test with bicarbonate solution, and a faint effervescence is detected. It looks like fatty acids are still
present, but I suppose even some soap, reformed during extraction, along with oxidized wazy ester, solid ester ( ethyl stearate is ).
Anyway, as usual I thank you all for attention, link to my YT video for a more detailed procedure and see you next time
palico
Reference
1. Fatty acids, their chemistry and chemical properties - Klare S. Markley, 1947, Interscience Publisher.
|
|
|
Texium
|
Thread Moved
27-3-2024 at 18:11 |
bnull
Hazard to Others
  
Posts: 167
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
What about transesterification with ethyl acetate? The solubility of fatty acids in EtOAc is about the same as in ethanol[1][2] and no
water is produced.
Did you test the wax? I mean, react it with sodium hydroxide to see if it was really ethyl stearate.
I suppose the yield could be higher if you had used more ethanol. You used 0.76 mole of fatty acids (assuming there were only oleic and stearic acids)
and 2.65 moles of ethanol. The ethanol could be increased to 4 moles (226 mL) or more to help dissolve the fatty acids.
Even so, it could end up being an ordeal as it seemed to be (I've watched the video). At least you got the orange oil in the end. Thanks for sharing.
[1] C. W. Hoerr, H. J. Harwood. "The Solubilities of Oleic and Linoeic Acids in Common Organic Solvents", https://doi.org/10.1021/j150501a008;
[2]. Rudi Heryanto et al. "Solubility of Stearic Acid in Various Organic Solvents and Its Prediction using Non-ideal Solution Models", http://dx.doi.org/10.2306/scienceasia1513-1874.2007.33.469.
[Edited on 28-3-2024 by bnull]
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Dear bnull, yes transesterification of ethyl acetate is a possible solution, it is discussed in my reference. I am not sure though if the emulsion
problem will not be represent, if the reaction is workupped as I did.
I did not use more ethanol simply because the flask was almost full. My plan was to employ 200 ml.
|
|
|
bnull
Hazard to Others
  
Posts: 167
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
I browsed the reference you provided. There is, in the page after the one that appears in your video (p. 270), a paragraph that describes the same
situation you were in. It begins with
| Quote: | | When the esterification mixture contains any apreciable amount of unreacted fatty acids, troublesome emulsions are often encountered if sodium or
potassium hydroxide or carbonate is used in an effort to remove them. |
The solution offered[1] involved the use of ethylene dichloride, which is highly inflammable, toxic, and possibly carcinogenic. I suppose
you could diesel instead. After all, sooner or later the biodiesel would be mixed with diesel.
[1] L. O. Buxton, R. Kapp. J. Am. Chem. Soc., 62, 986 (1940) . (I really love it when the first page of the paper is
the whole paper.)
[Edited on 31-3-2024 by bnull]
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2667
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I would encourage you to try the NaOMe reaction with triglycerides (fat) sometime to see how that works. It is not trivial, but can be done to near
completion OK. Using Ethanol with base is very tough, the rate of reaction is 10% of methanol, and you need a much larger excess of alcohol to make
it work. But one day I can see people making their own fuel from their waste products and crops. If we could find a way to better catalyze the
ethyl ester formation, it would be great, but much harder currently. Or better yet, design some yeast or plant to just make it for us.
Also need to design a plant that would make lots of oil per acre that would grow well in the US. Lots of tropical plants do it well, and Canola does
it OK in Canada, but soybeans don't make near as much oil as either warmer or cooler climate plants.
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Dear bnull, as solution I offered to distill of any volatiles before extracting residual fatty acids. I think it should reduce the emulsion.
In the video and the reference is said it is better to use a solvent for the reaction, one who can mix both ethanol and fatty acids. Yes, naphtha or
diesel maybe could.
Dear Dr. Bob, the basic recipe with methanol it is already super studied. I have ever looked for an alternative and I found in the direct transesterification.
Methanol is not readily available everywhere and basic recipe sensitive to free acids.
[Edited on 1-4-2024 by palico]
|
|
|
|