Toluene
Harmless

Posts: 26
Registered: 19-5-2013
Member Is Offline
Mood: No Mood
|
|
Would Fe(OH)3 + Acetic acid give me Iron (III) acetate?
Hi folks!, this evening I'm planning to experiment something, I have already done a little bit of FeCl3, so I'm thinking about react it with KOH
FeCl3 + 3KOH--> Fe(OH)3 + 3KCl
1) Would be a problem to separate de hydroxide from the potassium chloride?
2 Once I have the Fe(OH)3, can I mix it with pure glacial acetic acid to prepare this complicated molecule?
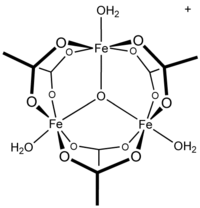
Could anyone please help me with the stecheometry for this reacction?
Fe(OH)3 + C2H4O2 -->[Fe3O(OAc)6(H2O)3]
Thanks a lot!!
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
1 no Fe(OH)3 is insoluble, but you need a good filter.
2 Yes
|
|
|
Toluene
Harmless

Posts: 26
Registered: 19-5-2013
Member Is Offline
Mood: No Mood
|
|
Thanks!, so would be a good Idea to add enough water to the mixture Fe(OH)3+KCl in order to dissolve all the KCl and filtrate the Fe(OH)3?
|
|
|
homeIandsecurity
Banned: Another incarnation of PhDChemist
Posts: 11
Registered: 30-11-2013
Member Is Offline
Mood: ready to put somebody in jail today
|
|
no! Because iron acetate isn't soluble in acetic acid...water must dissolve it.
And it is easy to test it...if iron hydroxide dissolves in acetic acid (aq), then it works.
And for this last question...forget about water molecule...3 acetate ions are needed for iron.
HOMELANDSECURITY@INTERPOL.US
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Absolutely use water. Be warned some iron acetate will go through your filter though.
[Edited on 30-11-2013 by bismuthate]
|
|
|
Toluene
Harmless

Posts: 26
Registered: 19-5-2013
Member Is Offline
Mood: No Mood
|
|
By the way, how does Iron acetate (III) look like?
|
|
|
shaheerniazi
Hazard to Self
 
Posts: 70
Registered: 25-11-2013
Location: Pakistan
Member Is Offline
Mood: radioactive
|
|
I once put Iron(III)Oxide in vinegar, and it fized did that produce iron acetate?
|
|
|
homeIandsecurity
Banned: Another incarnation of PhDChemist
Posts: 11
Registered: 30-11-2013
Member Is Offline
Mood: ready to put somebody in jail today
|
|
Maybe you put Iron(2) carbonate. How do you know it is Iron(3) oxide.
HOMELANDSECURITY@INTERPOL.US
|
|
|
shaheerniazi
Hazard to Self
 
Posts: 70
Registered: 25-11-2013
Location: Pakistan
Member Is Offline
Mood: radioactive
|
|
Because I bought it from a paint shop and it said Iron(III)oxide.
|
|
|
homeIandsecurity
Banned: Another incarnation of PhDChemist
Posts: 11
Registered: 30-11-2013
Member Is Offline
Mood: ready to put somebody in jail today
|
|
Maybe there was calcium carbonate made by exposure of paint (calcium hydroxide) to atmosphere...it is used as binder...iron is only used as a color.
HOMELANDSECURITY@INTERPOL.US
|
|
|
Toluene
Harmless

Posts: 26
Registered: 19-5-2013
Member Is Offline
Mood: No Mood
|
|
By the way, just mixing Fe(OH)3 + acetic acid will give me the desired product iron III acetate? or do I have to put the mixture under reflux?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
You will not have to reflux it, especially if the iron hydroxide is freshly precipitated. It may, however, take some time to react.
Also, the structure you drew (and the product of the reaction, according to Wikipedia) is not "true" iron(iii) acetate but a basic iron acetate
complex. Remember to adjust your stoichiometry accordingly.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I will not try using KOH, or even K2CO3 or a bicarbonate. I would expect the formation of a basic iron salt. As a source, Wikipedia
(http://en.wikipedia.org/wiki/Iron(III)_acetate ), on Iron(III) acetate to quote:
"Ferric acetate is the coordination compound more commonly known as "basic iron acetate". With the formula [Fe3O(OAc)6(H2O)3]OAc (OAc is CH3CO2-), it
is a salt, composed of the cation [Fe3(μ3-O)(OAc)6(H2O)3]+ and an acetate anion.[4] "
and further:
"Basic iron acetate forms on treating aqueous solutions of iron(III) sources with acetate salts.[6] Solid Iron may be mixed with hydrogen peroxide to
form Iron (II and/or III) Hydroxide, which may then be reacted with vinegar/acetic acid or acetate salts."
For an interesting discussion of many Iron salts, I would suggest Atomistry.com (link: http://iron.atomistry.com/ferric_chloride.html and links supplied there to other Iron salts together with comments at http://iron.atomistry.com/iron_salts.html ).
[Edited on 6-12-2013 by AJKOER]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Most commercial grades of oxides don't dissolve in acids, not even strong and concentrated ones, easily, sometimes almost not at all. That's
because the products have usually been calcinated at high temperature and for prolonged periods, strongly reducing their 'activity' towards acids.
Your Fe2O3 won't dissolve even in boiling glacial acetic acid to an appreciable degree.
Freshly precipitated iron (III) hydroxide however will dissolve in it easily, even at RT.
So, from a not to strong solution of a ferric salt, precipitate Fe(OH)3 with a stoichiometric amount of preferably strong ammonia, filter off the
Fe(OH)3 and wash it repeatedly with small amounts of water. Note that Fe(OH)3 has an annoying tendency to peptise.
Dissolve the filter cake in a small excess of glacial acetic acid, this is your ferric acetate (basic, I think) solution. To crystallise that salt may
prove the hardest bit, though... Most ferric salts are extremely water soluble.
[Edited on 7-12-2013 by blogfast25]
|
|
|