| Pages:
1
..
7
8
9
10 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Iron isn't a compound.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | You may not have noticed, but the rules of pretty much any game make it harder and more interesting.
Also, since they force you into thinking of unconventional solutions to the problem, they improve the intellectual content of the work done.
|
No, they don't.
Quote: Originally posted by unionised  | for example,, while I'm in 2 minds about whether the Mo blue is a polymer or not, at least including it for discussion led me to some interesting
stuff about big Mo clusters.
If it was just a matter of "search the web for the heaviest protein you can find" it's not interesting at all.
|
Just searching the web for the heaviest protein isn't synthesis.
The compounds that have been entered so far, including 'Mo Blues', have also been looked up on the Internet. So far the leader is a compound that's a
standard in the detection of phosphates, just how mind-bogglingly 'interesting' is that?
You keep making my case for me. Much obliged!
[Edited on 7-12-2014 by blogfast25]
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
So, you think that, for example, they banned the use of performance enhancing drugs in sport because they made people run slower?
They stop you using your hands in soccer because it's more difficult to pick up something and take it somewhere than to kick it and get it to land in
the same place?
(That must make mealtimes at your house interesting).
The idea is absurd- if there is a rule it is there because, otherwise, people would do what the rule forbids. You don't need a rule that bans hitching
a lift on the back of a tortoise in a running race. But there is a rule that stops you using a bicycle.
The rules stop you doing things the easy way (or,as it's referred to in that context, "cheating".
The fact that Mo blue has been in use for ages isn't very interesting. So, there wan't much point in you mentioning it- most of us will have already
known.
The structure of the stuff is interesting
Did you somehow come to the conclusion that they knew the structure when they used it for checking phosphates in plants and such?
Synthesising the biggest protein you can find isn't very interesting either.
Incidentally, perhaps you can explain how, if I buy or make - for example UHMW polythene you can guarantee that there are no short chains in it.
what you end up with is a probability distribution and, while it tends to zero for very hi and lo MW, it never quite gets there.
Anyway, if you have got your pants all twisted up by the idea of someone winning by making MO blue, do something a bit more clever, and win. How about
this?
http://www.chem.gla.ac.uk/cronin/publications/papers/2009/15...
[Edited on 7-12-14 by unionised]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
How would I go about making a polymer with an exact formula. You need to give an exact formula with a submission.
Should we let football (or soccer) be played with hands because only using feet limits the game?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | | So, you think that, for example, they banned the use of performance enhancing drugs in sport because they made people run slower?
|
Minor point, but the relative ease of competition is not why performance enhancing drugs are banned. Performance enhancing drugs are banned due to
perceived issues of artifice versus natural ability/training, or the concern of purchasing an advantage through pharmacological means rather than
dedication and genetic lot, in my opinion. You see technological advances such as use of triggers or new bow materials for archery allowed in many
competitions. Golf club material or weight isn't really regulated either, though where men and women start from is standardized as a rule, which
doesn't necessarily impact course difficulty.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Unionised:
Keep making ever more far fetched analogies and getting nowhere.
The truth is far more simple: creating an actual polymer doesn't give you an advantage in terms of easiness, it's by no means cheating. Comparing this
competition to a game or a sport is absurd. Let the best guy win, ferchrissake, not the one who manages to comply to your petty rules!
Polymers are interesting things to make, high MW and far more interesting than e.g. ammonium P molybdate. Or Mo Blue, but at least that one has the
redeeming feature of being truly high MW.
"Incidentally, perhaps you can explain how, if I buy or make - for example UHMW polythene you can guarantee that there are no short chains in
it."
That just shows how little you know about commercial polymers.
'Pants twisted'? Dear G-d. How about YOU do something interesting, huh?
[Edited on 7-12-2014 by blogfast25]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Yet... you still don't actually explain how to make a polymer with a specific formula.
[Edited on 7-12-2014 by bismuthate]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bismuthate  | Yet... you still don't actually explain how to make a polymer with a specific formula.
[Edited on 7-12-2014 by bismuthate] |
And you still have a compound in the competition that doesn't even exist!
Polymers HAVE a specific formula, unlike your non-existent antimony thingy.
[Edited on 7-12-2014 by blogfast25]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
No, I acknowledged that the references to this compound's existence weren't strong enough so I replaced my entry.
You seem to be taking this very personally.
And haven't answered my question.
[Edited on 7-12-2014 by bismuthate]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bismuthate  | No, I acknowledged that the references to this compound's existence weren't strong enough so I replaced my entry.
You seem to be taking this very personally.
And haven't answered my question.
[Edited on 7-12-2014 by bismuthate] |
Where?
Stop mind reading. You're not good at it.
I was arguing my case. Nothing more. I'm done with it now.
Good luck with the competition.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Ok. Can a mod cut the debate part out?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It really would be better to have competitions OFF of the 'Chemistry in General' topic.
My apologies for posting mine here.
Could a Mod please move mine to a new Competitions topic, or Beginnings, in order to prevent the relentless 'top of list' syndrome.
Edit:
Perhaps the general populace could chime in and say what they would prefer, en-masse, rather than just the few.
[Edited on 7-12-2014 by aga]
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Unionised:
Keep making ever more far fetched analogies and getting nowhere.
The truth is far more simple: creating an actual polymer doesn't give you an advantage in terms of easiness, it's by no means cheating. Comparing this
competition to a game or a sport is absurd. Let the best guy win, ferchrissake, not the one who manages to comply to your petty rules!
Polymers are interesting things to make, high MW and far more interesting than e.g. ammonium P molybdate. Or Mo Blue, but at least that one has the
redeeming feature of being truly high MW.
"Incidentally, perhaps you can explain how, if I buy or make - for example UHMW polythene you can guarantee that there are no short chains in
it."
That just shows how little you know about commercial polymers.
'Pants twisted'? Dear G-d. How about YOU do something interesting, huh?
[Edited on 7-12-2014 by blogfast25] |
OK, so it seems you are happy that the guy on a motorbike wins (we are disregarding the "petty rule" that it's a running race.
My analogy may be strained but others seem to understand it.
Anyway, back somewhere near the topic, (and since a few of us have asked) how do you make a polymer with a defined formula?
For example, how do you make polystyrene that is exactly (C7H8)1000 and doesn't contain the 999mer or the 1001mer?
Can you?
If not, how could you enter the product into the competition? It doesn't have a single defined MW (it has at least two depending on the definition)
And you may be surprised how much I know about commercial polymers. Did it occur to you that I'm not asking because I want to find out; I'm asking how
you think it's possible so I can correct you?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I am curious about the polymer lengths from either perspective. It's definitely not my field, but I have spent time in a polymer lab watching people
work, and I remember making a graduate student very uncomfortable by accidentally betraying that his photovoltaic polymers weren't as long in chain
length as the PI intended. This was in infancy, obviously, and I would love to know conceptual stats on radical termination and quality control, or
however you guys are envisioning it.
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well, here's my two cents.
I didn't know that moly blues were such a large complex. To me their structure definitely is interesting.
I thought the omission of polymers for this comp was clear and sensible from the start. If you object to that ruling for some kind of philosophical
reason then tough. You aren't the one making the rules.
Blogfast, in my considered opinion you are taking this all a bit personally. Rador is giving a smattering of cash to someone of their choosing and I
am learning some chemistry. (The comp may or may not be fair and might come down to rule interpretation. But I think it is the challenge and the ideas
that are the draw card not the money or the robust nature of the rules.)
I support a new topic for comps.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quick mind bender:
Perhaps Molly Blue is not quite so stable as one may imagine.
Perhaps the components swap places continuously, and the Measurements made are merely averages.
Same holds true for all highly Complex systems.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Despite all the debate, has anyone made a polymer they want to submit?
If not, forget it.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
amazing
Quote: Originally posted by aga  | Quick mind bender:
Perhaps Molly Blue is not quite so stable as one may imagine.
Perhaps the components swap places continuously, and the Measurements made are merely averages.
Same holds true for all highly Complex systems. |
that is the kind of stuff i like. i wonder too.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Quick mind bender:
Perhaps Molly Blue is not quite so stable as one may imagine.
Perhaps the components swap places continuously, and the Measurements made are merely averages.
Same holds true for all highly Complex systems. |
Not really, no. There's no reason to suspect any swapping. The structure is now well understood.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Probably so.
Personally i prefer to imagine things as dynamic equilibria rather than static.
Fits better with nature to my eye.
E.g. the notion that the entirety of observed matter is just 1 sufficiently energetic particle moving impossibly fast in all dimensions, seems fairly
reasonable.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Here's my submission, it just crystallized today.
Donated : Yes
Compound : Borotungstic Acid
Molecular Formula : H5BW12O40*30H2O
Molecular Mass : 3402.49
Synthesis : Tunsten disulfide obtained as dry lube was heated to decomposition at 1250°C. The yellow powder obtained, WO3 was dissolved
in a stoichometric amount of NaOH and the solution was stirred overnight. The solution was then gravity filtered and a portion of the extraction was
taken. To this portion, a 10% excess of HCl and H3BO3 were added and the solution was filtered. Next the solution was put into a sealed bag with
sodium hydroxide and evaporated to dryness. Final Yield : 0.85g Borotungstic acid, Confirmed by crystal appearance and low melting point.
Note : The crystals are actually light grey. The camera didn't pick up the color well.
      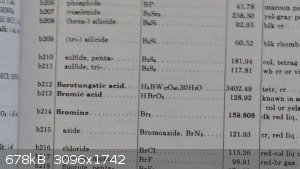  
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
The challenge ends at 0000 UTC! Submit your write-ups before then.
The current winner is gdflp with borotungstic acid (above).
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
I anticipated this would happen so I came prepared.
Barium phosphomolybdate
Ba3(PMo12O40)2
4056.9205 g/mol
Materials:
ammonium phosphomolybdate (made the way I posted earlier)
Ba(OH)2(Made from decomposing BaCO3 and adding the BaO to water)
Water
Vinegar
1 Make a solution by dissolving 1g of Ba(OH)2 in 50mL of hot water
2 Add .61g of ammonium phosphomolybdate to the solution and heat to about 60 degrees Centigrade for 5 minutes.
3 Ammonium gas is released and after all the yellow solid dissolves let the solution evaporate.
4 After it is evaporated add 50mL of cold vinegar to the solid and let it sit in an ice bath for 2 minutes.
5 Fliter, wash, and dry the slightly more orange solid.
2(NH4)3PMo12O40+3Ba(OH)2==>Ba3(PMo12O40)2+6NH3+6H2O
https://www.dropbox.com/sc/y1b471p9cwp2os3/AADDFBCm5cQMwUnYs...
[Edited on 15-12-2014 by bismuthate]
[Edited on 15-12-2014 by bismuthate]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
You missed the submission deadline though.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Oh crap I forgot It's UTC!
|
|
|
| Pages:
1
..
7
8
9
10 |