nowlan
Harmless

Posts: 6
Registered: 16-11-2014
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Steam Distillation advice
Hello,
Looking for some guidance on steam distillation.

I filled half of a 1000ml flask with petals, with aim of extracting the fragrance.
Filled the 500ml receiving flask with water and poured back into the first.
One of the issues I had was with the steam passing straight through the liebig condenser.
I believe it is 20cm or so from basic laboy kit. Over time I would see a lot of condensation occur near the vacuum take off adapter.
The liebig wasnt getting hot, but before and after were warm.
It did have some trouble going down the tube into the 500ml sometimes. I think it would just bead up, and kind of burp as it went down.
Currently I have around 200ml of hydrasol/distillate in the 500ml when I called it quits.
Has a horrible rotting fruit smell, like port wine etc. The original flowers had similar smell after being stored in amber bottle over night.
Originally had a green chlorophyll smell when i picked from plant.
Wondering what I should do next. I was expecting a layer of oil to develop on top of water. Believe it is a emulsion at the moment.
Should I try washing with calcium chloride (damprid) or sodium chloride (table salt)?
I realise I wont get much product. Think I need something thinner than sep funnel, like Burette.
Anthony.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
It's all in the hardware. That is your real issue.
Your oil is in the vapor, and it needs time, and reduced temp to separate properly.
I would save your emulsion, let it separate, and upgrade the gear you are using to suit the purpose.
https://www.youtube.com/watch?v=pSxvCM14lNo
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Cool video but I did not like the water flow in the condensor
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
LOL... Yeah. She had it backwards. Nice call.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
$399 + shipping for a 2 L steam distiller: the ideal gift from the 'perfect hubbie' to the 'bored housewife', I guess! 
(http://www.heartmagic.com/EssentialDistiller.html)
Still (no pun intended), better than this Blue Tack fan:
https://www.youtube.com/watch?v=r8-zxWKAVK8
Remember: "hot water always floats to the top!" 
[Edited on 1-4-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That might be a little too much water - try 1/2 that.
Did you use a steam injection tube (5-6mm glass tube) and did it reach all the way to the bottom of the pot? If not, try that.
Also I recommend using a steam trap just before the injection tube. This will separate out any entrained condensate from the steam. See picture in
my salicylaldehyde post.
Run the steam in as fast as your condenser can handle it.
Start with your receiver empty.
Heat the pot a little. Seems like you are already doing that.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I saw Mr. Fletchers Vid some time ago.
He indeed does create his own issues.
A proper steam distillation rig is on my radar... Seems a lovely way to pass an afternoon. Damn money!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by nowlan  | | Wondering what I should do next. I was expecting a layer of oil to develop on top of water. Believe it is a emulsion at the moment.
|
Volatile solvent for when the layer never layers, and sometimes even then.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I may be mistaken but I believe I read that sodium chloride (table salt) can help induce separation of emulsions. I believe you need to reach a
saturation level (of the salt) in the water.
Someone please correct this if I am wrong.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zombie  | I may be mistaken but I believe I read that sodium chloride (table salt) can help induce separation of emulsions. I believe you need to reach a
saturation level (of the salt) in the water.
Someone please correct this if I am wrong. |
Some emulsions can be tough to break. Really effing tough
|
|
|
nowlan
Harmless

Posts: 6
Registered: 16-11-2014
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Hello again,
@zombie, i dont think im doing true steam distillation. More boiling flowers in water, and letting steam take up oil across condenser into next flask.
Still not sure about the steam condensing after condenser. Is my problem that the condenser is too short? Should I boil the 3 neck softer? Steam is
passing through without touched the sides i suspect.
---
I had a go at trying to extract what ever was in the distillate today.
I setup a separation funnel and added my water.

I then poured around 50ml of diethyl ether into beaker.

I had some trouble with the separation funnel. Originally using a glass stopper except it started to leak. Do i need to grease the stopper too?
We ended up using a rubber stopper to get a better fit. After swirling the ether+water gas built up and ejected the rubber stopper like a champagne
cork across the room.

I noticed some beads appearing on the top of the separation funnel. not sure if these are the oils I was after or just a reaction between ether and
water.

I drained the lower aqueous layer into a beaker, leaving the ether in the funnel.

Notice that we lost some ether during separation. Closer to 40ml instead of original 50ml.

After pouring the ether layer into a 100ml beaker, we tried to boil it off using a vacuum pump and desiccator.


The vacuum pump caused the ether to evaporate, but chilled the beaker in the process. We then put it in a hot water bath, and tried once more.

After getting high breathing ether fumes coming from the vacuum pump, we checked what remained. Just under 20ml now.

After adding a little more ether, we decanted into a smaller 50ml beaker, and continued vacuum purge.


I continued boiling in hot water bath under vacuum again, until the beaker shifted/tiled due to being almost empty.

The final product after reducing down to almost nothing.

It no longer reeked of ether, but had more of a hay/wet grass smell.
The original aqueous solution from the separation funnel was stored back in the 500ml flask. It still contained the slightly off rotten fruit smell.
I am not sure how to reduce this down to essence. Would a slow boil stop it from pulling the smells with the steam?
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I think you boiled the flower petals too strongly. When you put them in the boiling flask like that, you can break up some of the nice smelling
compounds and lose what you're trying to get. It might work better to set up a dry steam distillation, where you boil your water separately, and lead
the steam through a tube into another vessel containing the petals. After the steam passes through the petals, it then goes into the condenser. It's
more complicated, but there's less risk of decomposition.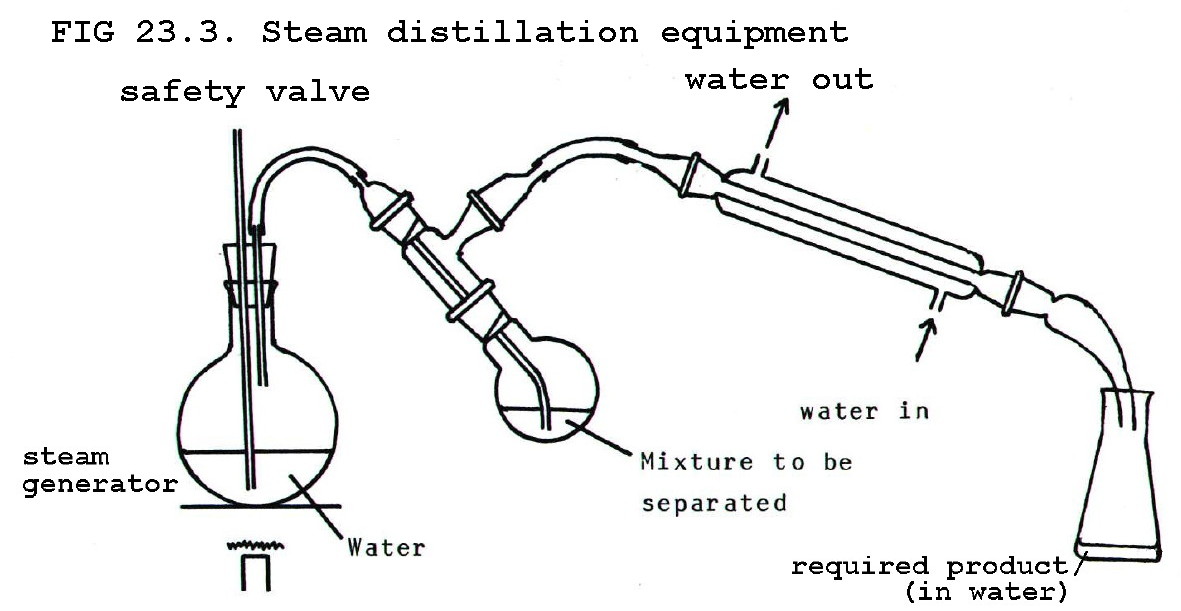
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Always vent through the stopcock after shaking until you get no more gas release. This is especially important with highly volatile
solvents like ether. With ether or DCM I will vent before shaking, or, after one quick shake, in the beginning.
Also, with DCM (probably w/ether too) I find it necessary to place a thin film of silicone grease on the glass plug to keep it from leaking. This is
somewhat dependant on how close a fit your particular separatory funnel/plug has, of course.
I have done many successful steam distillations with my 19/22 west condenser, which is 20cm. Running ice-water for coolant will help.
I agree with zts16 on water in the pot. Just run it dry and see how that works. It will load up with condensate soon enough anyway. But your
injection tube must run to the bottom of the pot. Otherwise how are you going to get the steam to the petals?
[Edited on 2-4-2015 by Magpie]
[Edited on 2-4-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Whether the steam source is internal or external makes no difference, except that an external source is more practical as you don't need to refill the
flask.
Nowlan, first of all, you are not considering the obvious. What is the plant species from which you got those petals? Have you checked the literature
to see if it contains sizeable amounts of essential oils at all? Flower petals rarely contain essential oils in useful concentrations. Their
fragrances are usually strongly smelling compounds that are rarely present in high enough amounts to be isolated by steam distillation. This is
without considering that some of these compounds decompose under steam distillation conditions, and that a lot of other cellular constituents
decompose to steam distillable compounds thus affecting the smell of the mixture.
Try something else than petals. Even with rose petals the majority of 2-phenylethanol and other components do not phase out of the distillate. You
should first try to use plant material that actually contains proper amounts of essential oils, especially if this is the first time you use this
technique.
Also, after you extract the essential oils with ether, you need to dry the ether solution, or else you cannot get the volatile components free of
water. Water is soluble in diethyl ether and will be taken to the product.
Another advice... don't ever evaporate bulk solvents in an desiccator, particularly not volatile solvents. You can have it boil over in the best of
cases and loose your product. A desiccator also has no heat source, so you cannot evaporate properly. You either use a rotavapor for solvent removal,
distil if working with larger amounts, or just improvise by evaporating from a flask equipped with magnetic stirrer and connected to an aspirator.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|