| Pages:
1
2 |
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I don't say that they don't exist (the CRC says that lead(II) cyanide will dissolve in KCN(aq), so presumably there is a cyano complex of lead(II) in
existence), but they won't show the great stability of transition metal complexes. The CRC doesn't list lead(IV) cyanide, which leads me to think
that the cyanide would be oxidized by it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
It's possible that Sb(CN)5 is not stable, yet that a mixed insoluble Sb(III) and Sb(V) cyanides might, nevertheless, be stable (if it forms at all).
That said, I do agree it's unlikely based on what we know, but woelen has said he'll try it out, so let's wait and see. I also agree
that formation of cyanogen is likely and that cyanide will not bond strongly to the metal as with transition metals.
[Edited on 14-4-2015 by deltaH]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I can give it a try on a small scale (tens of mg, in a test tube) and I am not scared of HCN or (CN)2 in such small amounts. If I have enough of the
dark blue compound with the chloride, then I could try adding a solution of KCN to the solid. I expect just formation of a white solid, but it is an
easy try, so why not?
Other experiments I want to try are
- adding a solution of N(CH3)4Cl to the golden yellow solution with antimony(III) and antimony(V)
- dissolving Sb2O5 in 40% HBr and dissolving Sb2O3 in HBr and mixing these solutions and then adding a solution of CsBr to it. I can imagine though
that the Sb2O5 could oxidize the HBr to Br2. I also need to make Sb2O5, I only have Sb2O3. I expect, however, that making Sb2O5 should not be too
difficult.
My first goal, however, is to obtain more of the dark blue solid. This evening I'll filter it and put it aside for drying.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | I can imagine though that the Sb2O5 could oxidize the HBr to Br2. I also need to make Sb2O5, I only have Sb2O3. I expect, however, that making Sb2O5
should not be too difficult.
|
Acc. these here reduction potentials (http://web.archive.org/web/20070518092613/http://www.northla...):
Br<sup>-</sup> to Br<sub>2</sub>(aq) = - 1.09 V
Sb<sub>2</sub>O<sub>5</sub> to Sb<sub>2</sub>O<sub>3</sub> (acid conditions) = + 0.65 V
So, no chance of Sb(V) oxidising bromide to bromine, at least acc. these values. (Sb(V) does oxidise iodide to iodine, as per my experience and SRP
values).
Also, based on the Cu(II) + cyanide == > copper(I) cyanide + cyanogen reaction, I think Sb(V) should also by strong enough to oxidise
cyanide to cyanogen.
For preparation of Sb<sub>2</sub>O<sub>5</sub>, I suggest preparing a HSbCl<sub>6</sub> solution from
Sb<sub>2</sub>O<sub>3</sub>, HCl and hydrogen peroxide (as per above). Then dilute a lot and neutralise with ammonia solution.
Sb<sub>2</sub>O<sub>5</sub> should precipitate.
[Edited on 14-4-2015 by blogfast25]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Some info about colour of Cs chloroantimonates:
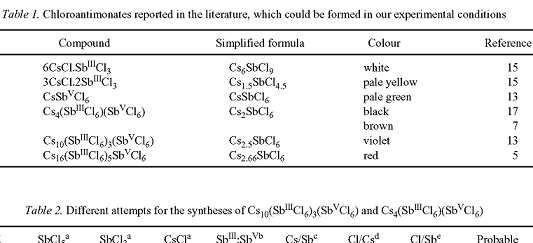
Some info about spectra Sb(III), Sb(V) and their mixture (in HCl(aq)):

Deeply coloured compounds can be also prepared with rubidium salts (Wienlan, Berichte, 1905). I tried [(n-Bu)4N]Cl, but only white
precipitate formed.
Potassium salt is golden-yellow.
Слава Україні !
Героям слава !
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@kmno4: Interesting info. Also interesting to see that there are other stoichiometries than the one I have seen in Pok's reference.
The color I have indeed can be described as black, but if you observe very carefully, then you can see that the material has a very dark purple/blue
color. You can best see this, if you keep the solid near a really black powder, such as carbon powder.
I tried to repeat the experiment with N(CH3)4Cl instead of CsCl, but this does not lead to a colored precipitate. I do get a precipitate, but it is
white. Probably it is either N(CH3)4SbCl4 or N(CH3)4SbCl6, with only antimony in oxidation state +3 or oxidation state +5, but not both.
I also did an experiment with the black solid by adding it to 60% HNO3. When this is done, then it quickly becomes white and a small amount of a
brown/orange gas can be observed (either NO2 or ONCl). The compound probably is oxidized by the nitric acid and it loses its chlorine, leaving Sb2O5
behind, which is known to be insoluble in nitric acid.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | Probably it is either N(CH3)4SbCl4 or N(CH3)4SbCl6, with only antimony in oxidation state +3 or oxidation state +5, but not both.
|
That can be verified by isolating and carefully washing the precipitate. Then prepare a little slurry of it in weak HCl and add strong KI solution to
it.
SbCl<sub>6</sub><sup>-</sup>/ SbCl<sub>4</sub><sup>-</sup> => Ered = + 0.75 V
I<sup>-</sup>/I<sub>2</sub> => Eox = - 0.54 V
So if the precipitate is the Sb(V) salt, iodine should form. I've used this test to differentiate between tin and antimony (Sn(IV) can't oxidise
iodide).
[Edited on 15-4-2015 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The final result of my synthesis of this compound is quite well. According to theory I should obtain just over 4.1 grams. I measured 3.9 grams of dark
material, which is a yield of better than 95%. I think that the reaction has nearly 100% yield (measured, based on amount of Sb2O3 used), due to the
very low solubility of the compound. The losses are mechanical.
I tested the remaining liquid after the material settled at the bottom. It does not show any opalescence on dilution with water. This means that it
must be nearly free of antimony. Even a tiny amount of Sb2O3, dissolved in HCl, gives a white opalescence on dilution with water. I kept the colorless
liquid, it is appr. 30 ml of concentrated HCl with a few tenths of grams of CsCl dissolved in it.
Below follow pictures of my 3.9 grams of dark powder:
 
Click the pictures for more detail. (The left picture is somewhat too blue, it was made in late afternoon daylight with blue sky, wityh the vial in
the shadow).
I'll make a webpage about this interesting experiment (probably next weekend).
[Edited on 15-4-15 by woelen]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I decided to check preparation (given by Wienland) of "(Sb, Sn)CI6(NH4)2".
Equal volumes of solutions of Sn(IV) and Sb(III/V) were mixed and nothing happened. Then some of solid NH4Cl was added, with continuous mixing : it
caused precipitation of black-violet ammonium complex salt. It is stable in strongly acidic media, water decomposes it to colourless products.
(both solutions of Sn and Sb were prepared from metals, HCl(aq) and calculated amounts of H2O2, to obtain ~0,1 mol Sn(IV) and ~(1+1) Sb(III)/Sb(V)
concentrations)
[Edited on 16-4-2015 by kmno4]
Слава Україні !
Героям слава !
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did the experiment with RbCl instead of CsCl, but this was not successful. When a solution of RbCl in conc. HCl is added to the golden yellow
liquid, mentioned above, then nothing seems to happen. No dark color is produced, no solid material appears. However, after several days of standing,
I have a small amount of a crystalline solid at the bottom, which is colorless/white.
-----------------------------------------------------------------------
For convenience I also ordered some Sb2O5, with the idea to do easier experimenting. But unfortunately, the Sb2O5 does not (or only very slowly)
dissolve in conc. HCl 
It is a pale yellow powder, which is remarkably inert. It does not dissolve in a hot solution of KOH, nor in hot conc. HCl.
I did an experiment by adding some Sb2O5 to conc. HCl and then heating it. It does not dissolve. Then I added some Sb2O3 to the still hot liquid. When
this touches the liquid there is a brief hissing noise and it dissolves at once. The color of the liquid is very pale yellow. The Sb2O5 still does not
dissolve.
Next, I added a few drops of a solution of CsCl in conc. HCl. This leads to formation of a white precipitate with a faint purplish/grey hue. So, only
a very small amount of the Sb2O5 dissolved in HCl.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Fusing the stubborn pentoxide with KOH or NaOH, followed by neutralisation of the antimonate should yield a more soluble form of the oxide. But you
already knew that, I'm sure. 
I'd also suggest to dissolve elemental Sb in aqua regia. It dissolves remarkably easily to HSbCl<sub>6</sub> (+5).
[Edited on 23-4-2015 by blogfast25]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I did the experiment with RbCl instead of CsCl, but this was not successful. When a solution of RbCl in conc. HCl is added to the golden yellow
liquid, mentioned above, then nothing seems to happen. No dark color is produced, no solid material appears... |
It is just a matter of concentrations. When Sb(III)/(V) concentration in solution is high (it is then coloured brown), both CsCl and RbCl give dark
violet precipitates. Cesium salt is easier to prepare. In paper by Wienland, rubidium salt is prepared via slightly different (and complicated) route
(the interested may bother to find and read the article)
Слава Україні !
Героям слава !
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@blogfast25: I tried dissolving in hot conc. KOH-solution, but this also does not work. Use of NaOH, according to literature, does not work, NaSbO3 is
not soluble in water, it is one of the very few insoluble sodium salts. I did not fuse it with KOH.
@kmno4: What concentration is needed for the dark brown color? With the reagents I have I tried making the liquid as concentrated as possible, but the
best I can reach is a golden yellow. If I add more Sb2O3 and H2O2, then the liquid becomes turbid. I have the impression that the dark brown solution,
which you mention, only can be obtained with SbCl3 in HCl and SbCl5 in HCl. Both SbCl3 and SbCl5, however, are not something you will find outside of
a lab in a home/amateur setting.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Fusing with KOH should work, I think (Holleman says so  ). Having said that, I
have some Cr2O3 that completely resisted fusing with KOH, not even the slightest bit of dissolution. Some oxide grades are very stubborn, as we know. ). Having said that, I
have some Cr2O3 that completely resisted fusing with KOH, not even the slightest bit of dissolution. Some oxide grades are very stubborn, as we know.
Yes, my Holleman confirms sodium antimonate to be insoluble. Used for sodium determinations.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I made videos of the component, while it is still on the filterpaper. These filter papers were used for preparing the powdered compound and after
scraping the solid from the paper, some material was left. I did an experiment with one of the filter papers, which was still wet with conc. HCl and
another experiment with the dried filterpaper, which was perfectly dry.
These videos nicely show that the dark compound quickly decomposes in the presence of water:
http://www.homescience.net/chem/exps/Sb_III_V/filter_still_a...
http://www.homescience.net/chem/exps/Sb_III_V/filter_dried.a...
The still acidic one decomposes slower, but it finally also completely decomposes.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It took some time, but I finally found the time to make a real webpage of the experiments with the mixed valency antimony compound:
http://woelen.homescience.net/science/chem/exps/Sb_III_V/ind...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Fabulous !
It is very hard to make any comment at all, due the sheer Awe inspired.
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Very seriously cool.
That caesium complex is very vivid in colour. Just amazing. Not something that I would have ever guessed.
And the disappearing black trick is amazing to watch.
|
|
|
| Pages:
1
2 |