Difference between revisions of "Quinine"
Diachrynic (Talk | contribs) |
|||
| (5 intermediate revisions by one other user not shown) | |||
| Line 8: | Line 8: | ||
| OtherNames = Chinine<br>Qualaquin<br>Quinate<br>Quinbisul | | OtherNames = Chinine<br>Qualaquin<br>Quinate<br>Quinbisul | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Quinine structure.png |
| − | | ImageSize = | + | | ImageSize = 250 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageCaption = Quinine structure |
| − | | ImageSize1 = | + | | ImageFile1 = Quinine_sulfate_solution.jpg |
| + | | ImageSize1 = 250 | ||
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| + | | ImageCaption1 = Fluorescence of an acidic aq. solution | ||
| ImageFile2 = | | ImageFile2 = | ||
| ImageSize2 = | | ImageSize2 = | ||
| Line 53: | Line 55: | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = Decomposes | | BoilingPt_notes = Decomposes | ||
| − | | Density = | + | | Density = 1.2 g/cm<sup>3</sup> |
| Formula = C<sub>20</sub>H<sub>24</sub>N<sub>2</sub>O<sub>2</sub> | | Formula = C<sub>20</sub>H<sub>24</sub>N<sub>2</sub>O<sub>2</sub> | ||
| HenryConstant = | | HenryConstant = | ||
| Line 128: | Line 130: | ||
===Physical=== | ===Physical=== | ||
| − | Quinine is a colorless or white solid, with a potent bitter taste. As freebase, it is insoluble in water, but more soluble in other organic solvents, such as [[chloroform]] or [[ethanol]]. | + | Quinine is a colorless or white solid, with a potent bitter taste. As freebase, it is insoluble in [[water]], but more soluble in other organic solvents, such as [[chloroform]] or [[ethanol]]. |
| + | |||
| + | ===Chromatgraphic=== | ||
| + | A variety of TLC conditions has been published to distinguish the cinchona alkaloids. Suitable systems on silica gel 60 are dichloromethane/diethylamine, v/v, 9/1, or chloroform/methanol/conc. ammonia solution, 17 + 2.8 + 0.2. Note that in order to observe the fluorescence of quinine, it may be necessary to spray the plate with ethanolic sulfuric acid.<ref name="illu" /> | ||
==Availability== | ==Availability== | ||
| Line 137: | Line 142: | ||
==Preparation== | ==Preparation== | ||
Quinine is best extracted from Cinchona tree bark than synthesized from precursors. | Quinine is best extracted from Cinchona tree bark than synthesized from precursors. | ||
| + | |||
| + | For extraction, the ground bark is mixed with slaked lime and some water and left to soak (it may be adviseable to carefully dry the mix?), followed by a soxhlet extraction with toluene.<ref>LambdaSyn, "Chinin aus Chinarinde", [https://www.lambdasyn.org/synfiles/chinin.html https://www.lambdasyn.org/synfiles/chinin.html]</ref> Purification is archived via the sulfate, because the (quinineH)<sub>2</sub>SO<sub>4</sub> salt has the lowest solubility of the cinchona alkaloid sulfates (1 g per 800 g water).<ref name="illu">lemmi, "Das Prochinin-Rätsel und die Chemie der Chinaalkaloide", [https://illumina-chemie.de/viewtopic.php?t=4527 https://illumina-chemie.de/viewtopic.php?t=4527]</ref> | ||
==Projects== | ==Projects== | ||
| Line 148: | Line 155: | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Exposure to quinine is known to cause abnormal heart rhythms. | + | Exposure to large amounts of quinine is known to cause abnormal heart rhythms. |
===Storage=== | ===Storage=== | ||
| Line 165: | Line 172: | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
[[Category:Biologically-derived compounds]] | [[Category:Biologically-derived compounds]] | ||
| + | [[Category:Fluorescent materials]] | ||
[[Category:Readily available chemicals]] | [[Category:Readily available chemicals]] | ||
Latest revision as of 18:36, 5 March 2024
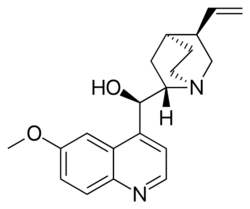 Quinine structure
| |
 Fluorescence of an acidic aq. solution
| |
| Names | |
|---|---|
| IUPAC name
(R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol
| |
| Other names
Chinine
Qualaquin Quinate Quinbisul | |
| Properties | |
| C20H24N2O2 | |
| Molar mass | 324.42 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.2 g/cm3 |
| Melting point | 177 °C (351 °F; 450 K) |
| Boiling point | Decomposes |
| 0.05 g/100 ml (15 °C) | |
| Solubility | Soluble in carbon disulfide, chloroform, ethanol Slightly soluble in glycerol Insoluble in ammonia, petroleum ether |
| Solubility in benzene | 1.25 g/100 ml |
| Solubility in chloroform | 83.3 g/100 ml |
| Solubility in diethyl ether | 0.4 g/100 ml |
| Solubility in ethanol | 125 g/100 ml |
| Solubility in glycerol | 5 g/100 ml |
| Vapor pressure | 1.54·10-10 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
1,800 mg/kg (guinea pig, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Quinine is an alkaloid isolated from Cinchona bark, used as medication to treat malaria and babesiosis.
Contents
Properties
Chemical
Quinine reacts with acids forming water soluble salts.
Quinine will slowly oxidize upon exposure to air over long periods of time, in both free and salt form, turning dark.
Physical
Quinine is a colorless or white solid, with a potent bitter taste. As freebase, it is insoluble in water, but more soluble in other organic solvents, such as chloroform or ethanol.
Chromatgraphic
A variety of TLC conditions has been published to distinguish the cinchona alkaloids. Suitable systems on silica gel 60 are dichloromethane/diethylamine, v/v, 9/1, or chloroform/methanol/conc. ammonia solution, 17 + 2.8 + 0.2. Note that in order to observe the fluorescence of quinine, it may be necessary to spray the plate with ethanolic sulfuric acid.[1]
Availability
Quinine is sold online. Often, it is sold in salt form, as sulfate.[2]
Tonic water has small amounts of quinine, though not enough for it to be an economical source.
Preparation
Quinine is best extracted from Cinchona tree bark than synthesized from precursors.
For extraction, the ground bark is mixed with slaked lime and some water and left to soak (it may be adviseable to carefully dry the mix?), followed by a soxhlet extraction with toluene.[3] Purification is archived via the sulfate, because the (quinineH)2SO4 salt has the lowest solubility of the cinchona alkaloid sulfates (1 g per 800 g water).[1]
Projects
- Compound collecting
- Bitter taste mixtures
- Tonic water
- Make fluorescent drinks
- Anti-malaria drug
- Demonstration of triboluminescence
Handling
Safety
Exposure to large amounts of quinine is known to cause abnormal heart rhythms.
Storage
Quinine should be kept in plastic or glass bottles, in dark places.
Disposal
Can be safely poured down the drain or in trash.
References
- ↑ 1.0 1.1 lemmi, "Das Prochinin-Rätsel und die Chemie der Chinaalkaloide", https://illumina-chemie.de/viewtopic.php?t=4527
- ↑ https://www.ebay.com/p/Quinine-Sulfate-Dihydrate-99-titration-Powder-50g/1648490218
- ↑ LambdaSyn, "Chinin aus Chinarinde", https://www.lambdasyn.org/synfiles/chinin.html