Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Nitric acid from mild electrochemistry
An article popped up in my news feed today and on reading this appears too good to be true.
https://www.nature.com/articles/s44160-023-00399-z
(Tldr: nitric acid produced with Fe2+ homogeneous catalyst at a graphite catalyst that is saturated with O2 and N2 at 0v. From the sounds of the
article powered by hydrogen consumption at a platinum electrode)
While a platinum electrode is on my wish list I'm incapable of replication. But this is a potentially valuable route if it's not BS. Any logic to calm
the hype or should we be hyped?
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I hate pay walls. Would love to try this, anything got to be better than my ostwald reactor. Stupid alcl3 keeps killing the catalyst
"You can't do that" - challenge accepted
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The "high" nitric acid production is ~8.5 mg per hour per gram of iron catalyst. Possibly a problem.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
So, a kilo of iron catalyst would bump production up to about 8 grams an hour. At 1.51g/mL, that would be around 5mL per hour, 24 hours a day, and 9
days for a liter.
Details, baby, im sure something killed their catalyst or slowed production or selectivity.
How didnthe catalyst not get converted into fe(no3)3.....
| Quote: | from Wikipedia
When dissolved, iron(III) nitrate forms yellow solutions. When this solution is heated to near boiling, nitric acid evaporates and a solid precipitate
of iron(III) oxide Fe
2O
3 appears.[7] |
Kinda solves that issue.
What specifically did they use as a catalyst?
"You can't do that" - challenge accepted
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Whats wrong with the more common methods using a nitrate salt and sulfuric acid?
You can use a bisulfate salt (pool PH-down) instead of the sulfuric acid if that is hard to get where you are located.
There are many procedures and videos about it online.
I like the old video from "extractions & ire" but it might not be around on the net anymore but you probably find it if search around a bit.
Wait, i look, here it is..
https://www.youtube.com/watch?v=VLOY-Jp2w1I
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Price, its hard to beat the cost if air.. nitrate salts are hard to find in my area, their here but hard to find and over priced.
So far i got cells to make/recover the reagents I use the most.
Electrolysis always seams to win in the end
Its cheaper for me to buy a 50lb bag of nh3cl and use it to make hcl, hno3 and al2o3,
but the setup is difficult to construct and maintain as only quartz can take the abuse.
Using a condencer at 220c is not fun anymore.
Ok, that was a lye, its still fun but im always looking for an easier method.
"You can't do that" - challenge accepted
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
I don't have access to the paper, but I downloaded their supplementary information, which has some hints.
It looks like they used ferrous sulfate for the catalyst, and the extended data showed that the faradic efficiency was greatest when the value of
ferrous sulfate was at 1. Unfortunately they didn't provide units, so I can only guess what that refers to (concentration?). It wouldn't be mol.
concentration, since their data went as high as 10, which is much higher than ferrous sulfate can be concentrated.
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Unfortunately I don't have access to the article through my university either. And it doesn't appear to be accessible through SciHub, yet. I wouldn't
expect this to be a very practical method though.
Edit: I will request an inter-library loan and see if I can get it that way.
[Edited on 10-13-2023 by Texium]
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Success.
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2696
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Thanks Texium! Typical rxn conditions are given as 1 mmol/L of FeSO4 in the caption of Figure 2. So that's 0.15 grams of iron sulfate per liter, or
0.056 grams of iron. So their reactor produces just under half a milligram of nitric acid per liter per hour. The paper also mentions that excessive
iron concentration reduces nitric acid production due to interaction of the catalyst with the key intermediate HO*. So, any takers? I think you might
be able to make a decent amount of nitric acid if you convert, say, a large fish tank, or a small swimming pool. But good luck isolating it. And the
reaction likes to be at pH 3, so if the concentration of HNO3 exceeds 0.001 M, you could have some problems.
I should mention I have some prior knowledge of electrocatalytic nitrogen fixation and I knew what to expect. Compared to other work in this field,
this is a very significant advancement and the researchers deserve a pat on the back. Compared to most actually practical methods of doing anything,
this is, um, well, you see 
[Edited on 13-10-2023 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I need a break from this nmr junk build ive dedicated the last 10 months to. Anything will be a relief from staring at datasheets failed fpga boards.
Besides, its almost that time of year again when I get to go panning, so im gonna be doing a lot of distillation anyway in preparation for this years
haul.
"You can't do that" - challenge accepted
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
Since it looks like the reaction relies on the formation of hydroxyl radicals, I did a quick search for other electrochemical methods of generating
them, and found this paper. Based on that, what about a porous carbon/titanium dioxide cathode with no iron required? If the carbon is porous enough, it might be
possible to bubble air straight through the cathode.
|
|
|
Texium
|
Thread Moved
14-10-2023 at 10:21 |
mysteriusbhoice
Hazard to Others
  
Posts: 475
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I got access to the full paper and sadly from what I read you need to run at 1.2V across the cell which means abysmal current density also they only
detected trace HNO3 and didnt do real production.
Heres my saved copy of the full paper
https://mega.nz/file/abxAlCoA#JgoE839FjTrcBNbkUckcsAWHWxKnFp...
Basically you need an entire jacuzzi of electrolyte and several m^2 of anode to run this at even 10A.
[Edited on 15-10-2023 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 475
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
If you want the TLDR of the paper here are the 2 relevant graphs for scale up.
Figure E is applied potential vs productivity which is in micro mols/hour(g).
Figure F red curve shows the applied potential for the best efficiency as shown in fig e and sadly if you look at current density you need one of
1.5ma/cm^2 which is.... CRAP and that 1.45V (anode cathode voltage diff) is what will get you 0V on cathode wrt to reference hydrogen electrode.
So yes sadly you will need a jaccuzi and lots of bubblers and meters of graphite foil anode for any meaningful production.
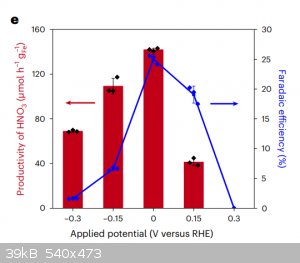
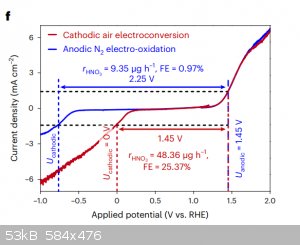
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Im gonna have to revisit some fundamentals to understand some of what your referencing, but i seen no exact details in the paper that i've seen in
other papers that make it easy to setup the same conditions they used.
Details such as membrane size/thickness and electrode spacing, these two parameters are determining factors in all my current electrolysis processes.
How important would the hydrogen reference electrode be in any attempt to recreate their results?
Obviously it will play some role as all their voltage potentials are measured from it as a common ground, but sence I do not have one right now, could
a model of the cell be worked out, without one?
Edit:
https://www.researchgate.net/publication/307883774
This paper describes in good detail the design, construction and usage of a Reversible Hydrogen Electrode
Should only cost a few dollars to construct. Hopefully platinum coated titanium will work.
[Edited on 15-10-2023 by Rainwater]
The paper also states that this is not a direct electrochemical reaction, but the in sute formation of H2O2 and OH-
which oxidizes N2.
All very confusing to me but what isn't at first. Hopefully I can have some time today to throw together a referance electrode and
test its response to different reactions. If I can get anything useful it will be the first project in many months with any amount of success
[Edited on 15-10-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
If you’re trying to chase something successful, I don’t think this is a good path to take. clearly_not_atara and mysteriusbhoice are right on in
their critiques. It’s a curiosity, not a practical method. Even if you did have a jacuzzi size reactor, the reaction would still shut down above
about 1 mM, about 63 mg nitric acid per liter… that’s nothing. Let’s say you somehow managed to set up a 1200 liter reactor. Well theoretically
you’d be able to get about 75 grams of nitric acid out of that. Maximum.
Maybe if you could figure out a way to slowly dose in potassium hydroxide or carbonate you could keep the pH adjusted to the ideal range and slowly
build up a more manageable concentration of potassium nitrate, but then you’d still have to do the normal distillation process to recover nitric
acid, and who knows, having a bunch of potassium nitrate in the solution might also screw up the electrolysis somehow.
Point is, in no way is it worth it— I don’t care what your signature says. 
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I think im getting the hang of the numbers now and see why, when the consentration of hno3 increases, the decomposition exceeds production
Basicly NO3- + 4H++ 3e- = NO + 2H2O; E0 = 0.96v
As the consentration of HNO3 increases, so does its reduction potential. Creating a equilibrium which limits the product to really low
amounts.
Without assistance from .... googling .... Le Chatelier's principles then you get stuck with very small amounts of product.
So now we need an insoluble nitrate salt that doesnt suffer from hydrolysis.
Guess ill stick with the reference electrode, should be easy enough and I get to play with fire.
"You can't do that" - challenge accepted
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
For those looking for nitric acid by electrolysis.
Here is a video by scrap science
https://youtu.be/AbrmHN5wOY8?si=BB9ZzbQerMYiZ9UF
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 475
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
for this version you can oxidize it using whatever anode really I will try with PbO2 and membrane is needed to prevent crossover of nitrate anion so a
cation membrane would help a lot. I also wanna try to go from urea to HNO3 but last time I did that I ended up with urea nitrate which samples went
pyrotechnic when cooking down!!
|
|
|
Stokes
Harmless

Posts: 7
Registered: 6-11-2023
Member Is Offline
|
|
Quote: Originally posted by Rainwater  | | I hate pay walls. Would love to try this, anything got to be better than my ostwald reactor. Stupid alcl3 keeps killing the catalyst
|
I know this is a bit off topic, but I would love to hear some details about your Ostwald reactor. I've never heard of aluminium chloride being a
problem before; where is that coming from?
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
I pipe the gas into a series of baby oil filled bubbler to filter out AlCl3 and unreacted NH4Cl and cool
Then its stright into a reactor based on this thread
https://www.sciencemadness.org/whisper/viewthread.php?tid=71...
But basicly NH4Cl + al + heat. -> AlCl3 + NH4Cl + NH3
The AlCl3 is only 50-80% unless you use a condencer at 200c to filter out the unreacted NH4Cl.
I was able to obtain pure samples but lots of work. Cl2 gas route is easier to do.
Quenching the dirty AlCl3 in water produces pure HCl gas and very fine mesh aluminum oxide while extracting the unreacted ammonia chloride.
All useful to me
"You can't do that" - challenge accepted
|
|
|
Stokes
Harmless

Posts: 7
Registered: 6-11-2023
Member Is Offline
|
|
Thanks for the information! That looks like a very interesting process.
If you want to selectively remove the aluminium chloride before the condenser stage, you could pass the reaction gas through a tube containing dry
sodium chloride. The aluminium chloride should react with the sodium chloride to form sodium tetrachloroaluminate, which is relatively stable, has a
fairly low vapor pressure, and melts at 157 ℃, which is very unusual for an ionic compound. It does still hydrolyse, though. I know Soviet chemists
used sodium chloride nozzles to remove aluminium chloride impurities from titanium tetrachloride gas streams, although I can't find a reference.
|
|
|