Carbon monoxide
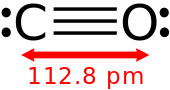 Structure of carbon monoxide, showing the triple bond between carbon and oxygen atoms.
| |
| Names | |
|---|---|
| IUPAC name
Carbon monoxide
| |
| Other names
Carbon monooxide
Carbon(II) oxide Carbonic oxide Carbonous oxide Carbonyl Exhaust gas Flue gas Monoxide | |
| Properties | |
| CO | |
| Molar mass | 28.010 g/mol |
| Appearance | Colorless gas |
| Odor | Odorless |
| Density | 789 kg/m3 (liquid) 1.250 kg/m3 (0 °C, 1 atm) 1.145 kg/m3 (25 °C, 1 atm) |
| Melting point | −205.02 °C (−337.04 °F; 68.13 K) |
| Boiling point | −191.5 °C (−312.7 °F; 81.6 K) |
| 27.6 mg/L (25 °C) | |
| Solubility | Soluble in glacial acetic acid, aq. ammonia, benzene, chloroform, aq. HCl-CuCl, ethanol, ethyl acetate, conc. HCl, methanol |
| Vapor pressure | 1.55·108 mmHg at 25 °C |
| Thermochemistry | |
| Std molar
entropy (S |
197.7 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−110.5 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −191 °C (−311.8 °F; 82.1 K) |
| Lethal dose or concentration (LD, LC): | |
| LC50 (Median concentration)
|
8,636 ppm (rat, 15 min) 5,207 ppm (rat, 30 min) 1,784 ppm (rat, 4 hr) 2,414 ppm (mouse, 4 hr) 5,647 ppm (guinea pig, 4 hr) |
| Related compounds | |
| Related compounds
|
Carbon dioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carbon monoxide is the inorganic chemical compound with the chemical formula CO, a colorless, odorless gas that is highly toxic to nearly all higher life-forms. Despite its inherent danger, it is a very useful and interesting reagent.
Contents
Properties
Chemical
Carbon monoxide burns if ignited in the presence of oxygen, forming carbon dioxide. At high temperatures and pressures it is reactive towards metals, forming metal carbonyls. In organic synthesis, carbon monoxide is a common method for carbonylating a variety of compounds in the presence of a metallic catalyst. It can be reacted with alkenes to form carboxylic acids, its hydrogenation produces methanol. Like carbon, at high temperatures it can reduce metal oxides to their elemental form.
Physical
Carbon monoxide is an acutely toxic, colorless, odorless gas at room temperature. It is very difficult to detect without special equipment.
Availability
Carbon monoxide is a common industrial chemical, but it is unlikely that it can be easily obtained by private citizens given its toxicity. It typically must be prepared in the lab. It can also form (extremely undesirably and dangerously for human life) when one stokes a wood or coal stove improperly, or keeps an automobile with a working engine in a closed garage.
Preparation
Carbon monoxide is most often produced by the incomplete combustion of organic materials that occurs without sufficient oxygen. However, it is most often produced for lab use by the dehydration of formic acid or oxalic acid using concentrated sulfuric acid.
- HCOOH → CO + H2O
- H2C2O4 → CO + CO2 + H2O
It can also be prepared in impure form by passing air or oxygen through burning coke or charcoal, and then once more through hot but non-burning charcoal, including volatile compounds and carbon dioxide as impurities. Passing carbon dioxide through hot coals also gives carbon monoxide. Passing water vapor through red hot coals, or the addition of water to them will give off a flammable mixture of carbon monoxide and hydrogen gas known as "water gas", a type of syngas.
- H2O + C → H2 + CO (ΔH = +131 kJ/mol)
Carbon monoxide can also be prepared by heating an equimolar mixture of calcium carbonate and zinc powder:
- CaCO3 + Zn → CaO + ZnO + CO
Being odorless and highly toxic, it's best to not make carbon monoxide at high concentrations without proper equipment and protection, and definitely not at all if you don't have any experience. Having a functional carbon monoxide detector present at all times is mandatory.
Projects
- Acetic anhydride synthesis
- Metal carbonyl synthesis
- Make sodium formate
- Make sodium oxalate
- Methanol synthesis
Handling
Safety
Carbon monoxide is very toxic and can easily knock a person unconscious due to oxygen starvation. If someone incapacitated this way is not quickly moved from the environment containing carbon monoxide, death is a near certainty. The symptoms of carbon monoxide poisoning are very easy to dismiss. One must be very careful using or attempting to store carbon monoxide; it is definitely recommended to use it only outside (a fume hood may not be sufficient, but if it must be used then a carbon monoxide detector is a a good idea). Acting irresponsibly with this chemical poses not only a threat to yourself, but to anyone else in the vicinity, so think twice before using it.
In case of carbon monoxide poisoning, the patient should immediately leave the contaminated room (or be carried from there) and given access to outside air. In case of severe poisoning, the patient should immediately receive medical attention. If no medical attention is available, the patient should be given pure oxygen to breathe, and methylene blue intravenously, which is a general antidote against blood agents that attack hemoglobin or cytochrome oxidase; the dosage is 50-100 ml of 1% solution. Other antidotes against carbon monoxide include cobinamide and acyzol (Russian anti-monoxide).
Storage
Storing carbon monoxide, both as compressed gas and as carbonyl compounds is dangerous, as in case of a leak, CO will rise and disperse well in the air (because its molar mass is similar to that of nitrogen. A carbon monoxide detector should be used in the immediate area of any potential release of any size.
Disposal
Carbon monoxide should only be released in air if there is no risk of build-up.