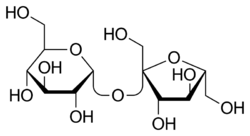
Sucrose
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
| |
| Other names
Cane sugar
Saccharose Table sugar | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.587 g/cm3 |
| Melting point | 186 °C (367 °F; 459 K) (decomposition) |
| Boiling point | Decomposes |
| 210 g/100 ml (25 °C) | |
| Solubility | Moderately sol in glycerol, pyridine Insoluble in acetone, diethyl ether, ethyl acetate |
| Solubility in ethanol | 0.6 g/100 ml |
| Solubility in methanol | 1 g/100 ml |
| Vapor pressure | ~0 mmHg |
| Acidity (pKa) | 12.6 |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
29,700 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Fructose Glucose |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sucrose, or as most know it, table sugar or even simply "sugar", is a disaccharide almost exclusively known as the primary artificial sweetener used in innumerable kinds of foods, though it actually is used in some chemistry settings. It may be referred to in historical literature as saccharose. It is comprised of fructose and glucose.
Contents
Properties
Chemical
Table sugar is so readily available and cheap in a very pure state that it can fill many different roles in chemistry where other chemicals could fit due simply to its extreme convenience. One use of sugar is in the production of high-purity carbon, which can be accomplished by decomposing it at very high temperatures without an open flame, or, for an even purer product, by evenly mixing fine sugar and concentrated sulfuric acid, dehydrating the sugar and leaving behind porous carbon that is nearly pure once washed.
Rather than burn on its own when heated in a flame, sucrose decomposes unevenly into a mixture of organic compounds, carbon, and water, commonly known as the confectionery caramel, accompanied by a pleasant smell. Solutions of sucrose, if heated too much, will also do this.
Sugar reacts vigorously with oxidizers when ignited, such as the popular "rocket candy" made of potassium nitrate and sugar used to power recreational model rockets. As this same reaction produces large amounts of thick white smoke without toxic effects or an unpleasant smell, that same mixture can be molded together to make smoke bombs. A mixture of potassium chlorate and sugar acts similarly with much less smoke; this mix can be ignited with a drop of concentrated sulfuric acid.
Sucrose is a disaccharide composed of equal parts glucose (dextrose) and fructose, two monosaccharides. Sucrose can be split into these two components by an extremely slow hydrolysis which is easily catalyzed by the addition of a weak acid such as potassium bitartrate (cream of tartar) or citric acid, and through mild heating.
As sugars are readily consumed by many yeasts and converted to alcohol by fermentation, sucrose is a useful starting point for the fermentation process that yields ethanol for use as a chemical reagent. Full guides on other websites detail the process of making ethanol that is safe to drink.
Physical
Sucrose is found in very pure form as the table sugar found in nearly every home in the industrialized world. It usually appears as fine transparent crystals of uniform size. It is extremely soluble in water, with 2 parts by mass of sugar dissolving in every one part water. Solutions of sucrose are notoriously sticky, and it is difficult to recrystallize sugar once it has been dissolved since heating will only convert it to caramel. As if this needs saying, sucrose is sweet. Very sweet.
Availability
Sucrose is found in nearly every supermarket, marketed as simply sugar or white sugar. Because nearly every product with the simple name is close to 100% sucrose, it is the most efficient money-wise to purchase it in large amounts.
Powdered sugar, raw sugar, brown sugar, and evaporated cane juice are all impure forms of sugar, containing cornstarch in the case of powdered sugar and derivatives of sugarcane in the rest. "Superfine" sugar, as opposed to granulated, is usually of the same composition but may cost more or usually contain agents to prevent caking.
Preparation
Sucrose isn't typically prepared by home chemists, considering its ease of access. Anyone with a food store nearby or internet access knows where to find it.
It can be prepared indirectly by growing sugar-rich plants in your backyard, such as sugar beet, or if you live at the tropics, sugarcane. Then there's just the matter of extracting the sugar from the harvested plants.
Projects
- Sugar and potassium nitrate smoke bombs
- Production of glucose for use as a reducing sugar
- Making rock candy, a fun introductory experiment for first-time chemists
- Make amorphous carbon
Handling
Safety
Sucrose, from a chemical standpoint, is one of the least toxic chemicals likely to be encountered in a lab. Many, many studies, with varying degrees and directions of bias, claim either that it is "healthy" or toxic, but regardless of those, it is not something that a home chemist should worry about being poisoned by while using it as a reagent.
Storage
Sugar is best stored in dry places, away from moisture to prevent fermentation and small animals like ants.
Disposal
Sugar can be poured down the drain or simply dumped in the trash or ground.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Biologically-derived compounds
- Carbohydrates
- Sugars
- Disaccharides
- Readily available chemicals
- Edible chemicals
- Materials available as food grade
- Solids