Difference between revisions of "Toluene"
Brain&Force (Talk | contribs) (Created page with "167x167px '''Toluene '''or '''methylbenzene '''is a clear, water-insoluble organic liquid, used as paint thinner and organic solvent. It is an a...") |
|||
| (17 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Chembox | |
| − | '''Toluene '''or '''methylbenzene '''is a clear, water-insoluble organic liquid, used as paint thinner and organic solvent. It is an [[aromatic hydrocarbon]]. | + | | Name = Toluene |
| + | | Reference = | ||
| + | | IUPACName = Toluene | ||
| + | | PIN = Toluene | ||
| + | | SystematicName = Methylbenzene | ||
| + | | OtherNames = Anisen<br>Phenylmethane<br>Toluol | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = Toluene-0.png | ||
| + | | ImageSize1 = 150 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless shiny liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 111 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 0.870 g/cm<sup>3</sup> (20 °C) | ||
| + | | Formula = C<sub>7</sub>H<sub>8</sub><br>C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 92.14 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −95 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Aromatic, "shoe glue"-like | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 0.052 g/100 ml (20 °C) | ||
| + | | SolubleOther = Miscible with glacial [[acetic acid]], [[acetone]], [[carbon disulfide]], [[chloroform]], [[diethyl ether]], [[ethanol]], [[hexane]], [[pyridine]], [[xylene]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 2.8 kPa (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 480 °C (896 °F; 753 K) | ||
| + | | ExploLimits = 1.1%-7.1% | ||
| + | | ExternalMSDS = [https://www.docdroid.net/mk4zP3s/toluene-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = 6 °C (43 °F; 279 K) | ||
| + | | LD50 = | ||
| + | | LC50 = >26,700 ppm (rat, 1 hr)<br>400 ppm (mouse, 24 hr) | ||
| + | | MainHazards = Flammable<br>Irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Benzene]]<br>[[Xylene]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Toluene '''or '''methylbenzene '''is a clear, water-insoluble organic liquid, used as paint thinner and organic solvent. It is an [[aromatic hydrocarbon]] with the general chemical formula '''C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub>'''. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | The methyl group makes toluene around 25 times more reactive than [[benzene]] in electrophilic aromatic substitutions. The addition of a halogen in the presence of UV light will yield benzyl halides. | + | The methyl group makes toluene around 25 times more reactive than [[benzene]] in electrophilic aromatic substitutions. The addition of a halogen in the presence of UV light will yield benzyl halides. |
| + | |||
| + | [[Potassium permanganate]] will oxidize toluene to yield [[benzoic acid]]. | ||
| + | |||
| + | : C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub> + [O] → C<sub>6</sub>H<sub>5</sub>COOH | ||
===Physical=== | ===Physical=== | ||
| − | Toluene is liquid at standard conditions, with a characteristic sweet, solvent-like smell. It is less dense than water, with a density of 0.87 g/ | + | Toluene is liquid at standard conditions, with a characteristic sweet, solvent-like smell. It is less dense than water, with a density of 0.87 g/ml. Toluene melts at -95 °C and boils at 111 °C. It is insoluble in water, but in vapor phase will form an azeotrope. Toluene is miscible with many organic solvents, like alcohols and esters. Toluene has a high refractive index, which causes it to exhibit iridescence. |
==Availability== | ==Availability== | ||
| − | Toluene is available at hardware as paint thinner, sometimes mixed with other hydrocarbons, depending on the product. Fractional distillation can be used to distill the components. | + | Toluene is available at hardware as paint thinner, sometimes relative pure, but most of the time is mixed with other hydrocarbons, ketones, depending on the product. [[Butanol]] and/or [[butyl acetate]] tends to be added most often in toluene-based solvents. Fractional distillation can be used to distill the components. Some solvents and thinners contain toluene mixed with water-miscible organics; technical grade toluene can be separated from such solvents by adding water. |
| + | |||
| + | Relative pure toluene can also be found in various shoe repair shops. | ||
==Preparation== | ==Preparation== | ||
There are a few ways to prepare toluene. One method involves the methylation of benzene with a methyl halide, such as methyl chloride in the presence of anhydrous [[aluminium chloride]]. Since toluene is less toxic than benzene, this is a good way, albeit consuming, to eliminate benzene. | There are a few ways to prepare toluene. One method involves the methylation of benzene with a methyl halide, such as methyl chloride in the presence of anhydrous [[aluminium chloride]]. Since toluene is less toxic than benzene, this is a good way, albeit consuming, to eliminate benzene. | ||
| + | |||
| + | However, making toluene in bulk through this method is not very practical and economical, and it's much cheaper to simply buy toluene from the store than to make it yourself. | ||
==Projects== | ==Projects== | ||
| − | *[[Benzoic acid]] | + | *[[Benzoic acid]] synthesis |
| − | *Benzaldehyde synthesis | + | *[[Benzaldehyde]] synthesis (Étard reaction) |
| + | *Chlorotoluenes | ||
| + | *[[Benzyl chloride]] synthesis | ||
*[[Mononitrotoluene|Mono]], [[Dinitrotoluene|di]] and [[trinitrotoluene]] | *[[Mononitrotoluene|Mono]], [[Dinitrotoluene|di]] and [[trinitrotoluene]] | ||
| − | *Sulfur extraction | + | *[[Sulfur]] extraction |
| + | *Hemoglobin extraction | ||
==Handling== | ==Handling== | ||
| Line 29: | Line 148: | ||
===Disposal=== | ===Disposal=== | ||
| − | Burning toluene will release soot, so it's best to | + | Burning toluene will release soot, so it's best to do this '''outside'''. Since the burning is incomplete, the resulting smoke will have plenty of soot, carbon monoxide as well as unburnt toluene vapors, giving the smoke a characteristic smell. If you're using this method, try not to burn too much toluene at once, and instead burn it in small amounts over a longer period of time. |
| + | |||
| + | Since it's an aromatic, [[Fenton's reagent]] can also be a good choice to destroy it. [[Talk:Toluene]] However, the said method produces lots of gasses which will aerosolize some toluene, if too much is added. This is dangerous in an enclosed area, carrying both a fire/explosion hazard as well as inhaling dangerous toluene fumes. This can be controlled by adding the toluene dropwise or bubbling (non-flammable) gas-carried toluene through a gas diffusing stone (make sure the toluene can pass through the frite) through the oxidizing solution to limit vapor escape. Either perform the neutralization in a fumehood, or outside. Doing it outside is desired, as UV light will catalyze the decomposition. If you want to use a photochemical neutralization, it's best to use a UV lamp. | ||
==References== | ==References== | ||
| Line 36: | Line 157: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=24592 Toluene from benzene, styrene, gasoline?] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=24592 Toluene from benzene, styrene, gasoline?] | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=2223 toluene --> benzaldehyde] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=2223 toluene --> benzaldehyde] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Organic compounds]] | ||
| + | [[Category:Hydrocarbons]] | ||
| + | [[Category:Aromatic compounds]] | ||
| + | [[Category:Fragrant compounds]] | ||
| + | [[Category:Solvents]] | ||
| + | [[Category:Nonpolar solvents]] | ||
| + | [[Category:Volatile chemicals]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Essential reagents]] | ||
| + | [[Category:DEA List II chemicals]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 19:16, 15 September 2022
| |
| Names | |
|---|---|
| IUPAC name
Toluene
| |
| Preferred IUPAC name
Toluene | |
| Systematic IUPAC name
Methylbenzene | |
| Other names
Anisen
Phenylmethane Toluol | |
| Properties | |
| C7H8 C6H5CH3 | |
| Molar mass | 92.14 g/mol |
| Appearance | Colorless shiny liquid |
| Odor | Aromatic, "shoe glue"-like |
| Density | 0.870 g/cm3 (20 °C) |
| Melting point | −95 °C (−139 °F; 178 K) |
| Boiling point | 111 °C (232 °F; 384 K) |
| 0.052 g/100 ml (20 °C) | |
| Solubility | Miscible with glacial acetic acid, acetone, carbon disulfide, chloroform, diethyl ether, ethanol, hexane, pyridine, xylene |
| Vapor pressure | 2.8 kPa (20 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 6 °C (43 °F; 279 K) |
| Lethal dose or concentration (LD, LC): | |
| LC50 (Median concentration)
|
>26,700 ppm (rat, 1 hr) 400 ppm (mouse, 24 hr) |
| Related compounds | |
| Related compounds
|
Benzene Xylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
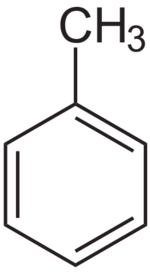
Toluene or methylbenzene is a clear, water-insoluble organic liquid, used as paint thinner and organic solvent. It is an aromatic hydrocarbon with the general chemical formula C6H5CH3.
Contents
Properties
Chemical
The methyl group makes toluene around 25 times more reactive than benzene in electrophilic aromatic substitutions. The addition of a halogen in the presence of UV light will yield benzyl halides.
Potassium permanganate will oxidize toluene to yield benzoic acid.
- C6H5CH3 + [O] → C6H5COOH
Physical
Toluene is liquid at standard conditions, with a characteristic sweet, solvent-like smell. It is less dense than water, with a density of 0.87 g/ml. Toluene melts at -95 °C and boils at 111 °C. It is insoluble in water, but in vapor phase will form an azeotrope. Toluene is miscible with many organic solvents, like alcohols and esters. Toluene has a high refractive index, which causes it to exhibit iridescence.
Availability
Toluene is available at hardware as paint thinner, sometimes relative pure, but most of the time is mixed with other hydrocarbons, ketones, depending on the product. Butanol and/or butyl acetate tends to be added most often in toluene-based solvents. Fractional distillation can be used to distill the components. Some solvents and thinners contain toluene mixed with water-miscible organics; technical grade toluene can be separated from such solvents by adding water.
Relative pure toluene can also be found in various shoe repair shops.
Preparation
There are a few ways to prepare toluene. One method involves the methylation of benzene with a methyl halide, such as methyl chloride in the presence of anhydrous aluminium chloride. Since toluene is less toxic than benzene, this is a good way, albeit consuming, to eliminate benzene.
However, making toluene in bulk through this method is not very practical and economical, and it's much cheaper to simply buy toluene from the store than to make it yourself.
Projects
- Benzoic acid synthesis
- Benzaldehyde synthesis (Étard reaction)
- Chlorotoluenes
- Benzyl chloride synthesis
- Mono, di and trinitrotoluene
- Sulfur extraction
- Hemoglobin extraction
Handling
Safety
Toluene vapors can cause headaches, dizziness, nausea, so work should be performed in a fume hood or outside. Toluene is less toxic and carcinogenic than benzene, meaning it can be used as a substitute for benzene as a solvent.
Storage
Toluene is less volatile than other hydrocarbons, but due to its strong smell it's best to store it in closed bottles, and kept in a solvent cabinet.
Disposal
Burning toluene will release soot, so it's best to do this outside. Since the burning is incomplete, the resulting smoke will have plenty of soot, carbon monoxide as well as unburnt toluene vapors, giving the smoke a characteristic smell. If you're using this method, try not to burn too much toluene at once, and instead burn it in small amounts over a longer period of time.
Since it's an aromatic, Fenton's reagent can also be a good choice to destroy it. Talk:Toluene However, the said method produces lots of gasses which will aerosolize some toluene, if too much is added. This is dangerous in an enclosed area, carrying both a fire/explosion hazard as well as inhaling dangerous toluene fumes. This can be controlled by adding the toluene dropwise or bubbling (non-flammable) gas-carried toluene through a gas diffusing stone (make sure the toluene can pass through the frite) through the oxidizing solution to limit vapor escape. Either perform the neutralization in a fumehood, or outside. Doing it outside is desired, as UV light will catalyze the decomposition. If you want to use a photochemical neutralization, it's best to use a UV lamp.