Acetaldehyde
| |
| Names | |
|---|---|
| IUPAC name
Acetaldehyde
| |
| Preferred IUPAC name
Acetaldehyde | |
| Systematic IUPAC name
Ethanal | |
| Other names
Acetic aldehyde
Ethyl aldehyde Acetylaldehyde | |
| Properties | |
| C2H4O CH3CHO | |
| Molar mass | 44.05 g/mol |
| Appearance | Colorless volatile liquid |
| Odor | Ether-like |
| Density | 0.7928 g/cm3 (10 °C) 0.784 g/cm3 (20 °C) |
| Melting point | −123.37 °C (−190.07 °F; 149.78 K) |
| Boiling point | 20.2 °C (68.4 °F; 293.3 K) |
| Miscible | |
| Solubility | Reacts with ammines Miscible with glacial acetic acid, acetone, benzene, diethyl ether, ethanol, ethyl acetate, gasoline, methanol, toluene, turpentine, xylene Slightly soluble in chloroform |
| Vapor pressure | 740 mmHg (20 °C) |
| Acidity (pKa) | 13.57 |
| Thermochemistry | |
| Std molar
entropy (S |
250 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−166 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −39 °C (−38.2 °F; 234.15 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
1,930 mg/kg (rat, oral) |
| LC50 (Median concentration)
|
13,000 ppm (rat) 17,000 ppm (hamster) 20,000 ppm (rat) |
| Related compounds | |
| Related compounds
|
Formaldehyde Propionaldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
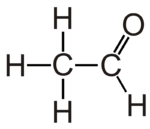
Acetaldehyde is the organic compound with chemical formula CH3CHO. It is the second-simplest aldehyde and finds use as a building block in organic synthesis.
Contents
Properties
Chemical
Depending on reaction conditions, the oxidation of acetaldehyde by oxygen variously co-produces acetic anhydride and acetic acid via the intermediate peracetic acid. The process relies on a catalyst containing metal ions and is typically conducted by introduction of gaseous oxygen into liquid acetaldehyde.
Physical
Acetaldehyde is a transparent, volatile, and extremely flammable liquid at room temperature that boils at only 20.2°C (68.4°F), making it difficult to store. The characteristic odor of acetaldehyde is sweet and reminiscent of green apple. Acetaldehyde has a flash point of only −39°C, making it absolutely crucial that any significant amount is kept away from possible ignition sources.
Availability
No over-the-counter source of acetaldehyde is known, and sources are unlikely due to the difficulty and danger of prolonged storage.
Preparation
Industrially, acetaldehyde is produced via the oxidation of ethene over a copper-palladium catalyst.
- 2 CH2=CH2 + O2 → 2 CH3CHO
Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C:
- 2 CH3CH2OH + O2 → 2 CH3CHO + 2 H2O
The second method of preparing acetaldehyde from ethanol involves its dehydrogenation at a temperature of of 260-290 °C, again over a catalyst of copper. This route is endothermic, requiring constant and uniform heating, but has the advantage of not requiring oxygen input, which also reduces the risk of fire.
- CH3CH2OH → CH3CHO + H2[1]
During the synthesis of dioxane from ethylene glycol using conc. sulfuric acid as catalyst, small amounts of acetaldehyde will be produced. However you will need to convert large amounts of ethylene glycol into dioxane to get a significant amount of acetaldehyde.
Projects
- Pentaerythritol synthesis
- Crotonaldehyde synthesis
Handling
Safety
Acetaldehyde is designated a probable carcinogen and is many times more toxic than ethanol, which it is a metabolite of. With a high chance of vaporization and a flash point of only −39 °C, acetaldehyde represents a significant fire hazard, and care should be made that all potential sources of ignition are kept away.
Storage
Acetaldehyde must be stored in a highly temperature-controlled environment given its low boiling point, and should also be kept in a separate cabinet or secondary container in a well-ventilated space, always away from potential sources of ignition.
Another option is to store it into a metal pressurized container, such as a lecture bottle.
Small amounts of niacinamide are sometimes used as stabilizer.[2]
Disposal
Acetaldehyde can be safely burned. Do this outside.
References
- ↑ Ullmann's Encyclopedia Of Industrial Chemistry, Wiley-VCH (2007)
- ↑ https://www.sigmaaldrich.com/catalog/product/sial/w200320