Difference between revisions of "Calcium oxide"
| (8 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
| IUPACName = Calcium oxide | | IUPACName = Calcium oxide | ||
| PIN = | | PIN = | ||
| − | | SystematicName = | + | | SystematicName = Calcium oxide |
| − | | OtherNames = Quicklime | + | | OtherNames = Calcium monoxide<br>Quicklime |
<!-- Images --> | <!-- Images --> | ||
| ImageFile = | | ImageFile = | ||
| Line 63: | Line 63: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = 12.8 | | pKa = 12.8 | ||
| pKb = | | pKb = | ||
| Solubility = Reacts | | Solubility = Reacts | ||
| − | | SolubleOther = Reacts exothermically with all acids, [[ketone]]s<br>Insoluble in [[ | + | | SolubleOther = Reacts exothermically with all acids, [[ketone]]s<br>Insoluble in [[alcohol]]s, [[ether]]s, halocarbons, hydrocarbons |
| Solvent = | | Solvent = | ||
| VaporPressure = ~0 mmHg | | VaporPressure = ~0 mmHg | ||
| Line 106: | Line 107: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = [[Magnesium oxide]]<br>[[Strontium oxide]] | + | | OtherCompounds = [[Magnesium oxide]]<br>[[Strontium oxide]]<br>[[Barium oxide]] |
}} | }} | ||
}} | }} | ||
| Line 116: | Line 117: | ||
===Physical=== | ===Physical=== | ||
| − | Calcium oxide is | + | Calcium oxide is an odorless fine white solid powder. It reacts violently with water and skin. It is insoluble in [[methanol]]. |
==Availability== | ==Availability== | ||
Calcium oxide can be bought at the construction or home improvement stores as quicklime or burnt lime in sacks or buckets. The ambiguous term "lime" usually refers to a mixture of calcium and/or magnesium compounds, though sometimes may serve as a label for pure calcium oxide. | Calcium oxide can be bought at the construction or home improvement stores as quicklime or burnt lime in sacks or buckets. The ambiguous term "lime" usually refers to a mixture of calcium and/or magnesium compounds, though sometimes may serve as a label for pure calcium oxide. | ||
| − | In many | + | In many locations, quicklime is no longer sold by most construction stores, mainly due to its hazards, and thus has become almost impossible to find. Even where it's available, old quicklime tends to be partially hydrolyzed, since it will slowly absorb moisture from air, and neutralize. This can also happen to quicklime kept in places with high humidity for short periods of time. |
==Preparation== | ==Preparation== | ||
| − | Calcium oxide is prepared from the thermal decomposition of [[calcium carbonate]] (lime), at temperatures over 825 °C. If the quicklime is not stable and after it cools, it will spontaneously react with CO<sub>2</sub> from the air, converting it back to calcium carbonate. It should also be performed in a dry environment to prevent hydrolysis back into [[calcium hydroxide]]. | + | Calcium oxide is prepared from the thermal decomposition of [[calcium carbonate]] (lime), at temperatures over 825 °C. If the quicklime is not stable and after it cools, it will spontaneously react with CO<sub>2</sub> from the air, converting it back to calcium carbonate. It should also be performed in a dry environment to prevent hydrolysis back into [[calcium hydroxide]]. Various sources of calcium carbonate, like eggshells and snail shells can be used.<ref>https://www.youtube.com/watch?v=Ek3aeUhHaFY</ref> |
| − | Quicklime is best synthesized in bulk, in lime kilns. | + | Quicklime is best synthesized in bulk, in lime kilns. Bulk limestone works better than powdered calcium carbonate, as heat is transferred better through the mass and little calcium oxide gets blown in the smoke. The high surface of powdered calcium carbonate/hydroxide will also mean that the resulting calcium oxide will absorb water or carbon dioxide from air much faster than the bulk form. |
| − | Calcium hydroxide can also be dehydrated to calcium oxide by heating it at 580 °C for a few hours. This reaction is generally preferred, as it takes place at lower temperatures, which are much easier to attain. | + | Calcium hydroxide can also be dehydrated to calcium oxide by heating it at 580 °C for a few hours. This reaction is generally preferred, as it takes place at lower temperatures, which are much easier to attain. As mentioned before, powdered calcium hydroxide does not convert as good as bulk form. |
In both cases, after the reaction has completed, the hot quicklime should be left in a container filled with a neutral gas, such as [[nitrogen]], to prevent the oxide from reacting with water vapors or carbon dioxide. [[Oxygen]] can also be used. If synthesized in large amounts, this is not required, as only the surface oxide will convert to limestone, which acts as a protective layer for the inner quicklime. | In both cases, after the reaction has completed, the hot quicklime should be left in a container filled with a neutral gas, such as [[nitrogen]], to prevent the oxide from reacting with water vapors or carbon dioxide. [[Oxygen]] can also be used. If synthesized in large amounts, this is not required, as only the surface oxide will convert to limestone, which acts as a protective layer for the inner quicklime. | ||
| − | If the temperature is high enough, the calcium oxide will harden into fragile chunks, which can be easily separated from the leftover calcium carbonate/hydroxide by sifting it through a sieve. | + | If the temperature is high enough, the calcium oxide will harden into fragile chunks, which can be easily separated from the leftover calcium carbonate/hydroxide by carefully sifting it through a sieve. |
==Projects== | ==Projects== | ||
| Line 139: | Line 140: | ||
*Desiccator | *Desiccator | ||
*Make calcium salts | *Make calcium salts | ||
| − | *Make calcium carbonate | + | *Make [[calcium carbonate]] |
| − | *Make calcium cyanamide | + | *Make [[calcium cyanamide]] |
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Calcium oxide is extremely corrosive to the human tissue as it reacts vigorously with the water from | + | Calcium oxide is extremely corrosive to the human tissue as it reacts vigorously with the water from tissues, releasing lots of heat, which causes severe burns. Quicklime is not normally a fire hazard, but sometimes its reaction with water can release sufficient heat to ignite combustible materials. Neutralizing calcium oxide even with a weak acid will release copious amounts of heat. Intentionally mixing calcium oxide with water in a controlled and well-thought out setup can be used to produce the somewhat more stable [[calcium hydroxide]]. Gloves, face mask and goggles should be worn when handling the powder, as most the commercial powder is extremely fine and can easily become airborne. |
===Storage=== | ===Storage=== | ||
| − | Calcium oxide should be stored in closed or sealed bottles, in a dry place, to prevent it from absorbing water and carbon dioxide from air. | + | Calcium oxide should be stored in closed or sealed bottles, in a dry place, to prevent it from absorbing water and carbon dioxide from air. Small amounts of CaO will rapidly degrade in contact with air, while larger amounts take much longer. |
===Disposal=== | ===Disposal=== | ||
| − | Calcium oxide should be neutralized before being discarded of. It doesn't pose any hazards to the environment after being neutralized and can be safely dumped in the ground. Avoid dumping it on grassland as it will burn it. | + | Calcium oxide should be neutralized before being discarded of. It doesn't pose any hazards to the environment after being neutralized and can be safely dumped in the ground, preferably construction sites. Avoid dumping it on grassland as it will burn it. Dumping it in trash is also an alternative. |
==References== | ==References== | ||
| Line 170: | Line 171: | ||
[[Category:Irritants]] | [[Category:Irritants]] | ||
[[Category:Air-sensitive materials]] | [[Category:Air-sensitive materials]] | ||
| + | [[Category:Ceramic materials]] | ||
Latest revision as of 23:42, 20 March 2020
 CaO sample
| |
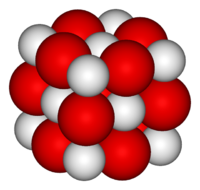 CaO molecular structure
| |
| Names | |
|---|---|
| IUPAC name
Calcium oxide
| |
| Systematic IUPAC name
Calcium oxide | |
| Other names
Calcium monoxide
Quicklime | |
| Properties | |
| CaO | |
| Molar mass | 56.0774 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 3.34 g/cm3 |
| Melting point | 2,613 °C (4,735 °F; 2,886 K) |
| Boiling point | 2,850 °C (5,160 °F; 3,120 K) |
| Reacts | |
| Solubility | Reacts exothermically with all acids, ketones Insoluble in alcohols, ethers, halocarbons, hydrocarbons |
| Vapor pressure | ~0 mmHg |
| Acidity (pKa) | 12.8 |
| Thermochemistry | |
| Std molar
entropy (S |
40 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−635 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds
|
Magnesium oxide Strontium oxide Barium oxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium oxide, also known as quicklime or burnt lime, is a white, caustic, alkaline chemical compound, mostly used in construction. It has chemical formula CaO.
Contents
Properties
Chemical
Calcium oxide can be reacted, or slaked, with water, releasing large amounts of heat and producing calcium hydroxide. In open air, it reacts with water vapor and carbon dioxide, slowly converting to calcium carbonate over time. It is used as a component of many cement mixes, and can be reacted with acids to form calcium salts.
Physical
Calcium oxide is an odorless fine white solid powder. It reacts violently with water and skin. It is insoluble in methanol.
Availability
Calcium oxide can be bought at the construction or home improvement stores as quicklime or burnt lime in sacks or buckets. The ambiguous term "lime" usually refers to a mixture of calcium and/or magnesium compounds, though sometimes may serve as a label for pure calcium oxide.
In many locations, quicklime is no longer sold by most construction stores, mainly due to its hazards, and thus has become almost impossible to find. Even where it's available, old quicklime tends to be partially hydrolyzed, since it will slowly absorb moisture from air, and neutralize. This can also happen to quicklime kept in places with high humidity for short periods of time.
Preparation
Calcium oxide is prepared from the thermal decomposition of calcium carbonate (lime), at temperatures over 825 °C. If the quicklime is not stable and after it cools, it will spontaneously react with CO2 from the air, converting it back to calcium carbonate. It should also be performed in a dry environment to prevent hydrolysis back into calcium hydroxide. Various sources of calcium carbonate, like eggshells and snail shells can be used.[1]
Quicklime is best synthesized in bulk, in lime kilns. Bulk limestone works better than powdered calcium carbonate, as heat is transferred better through the mass and little calcium oxide gets blown in the smoke. The high surface of powdered calcium carbonate/hydroxide will also mean that the resulting calcium oxide will absorb water or carbon dioxide from air much faster than the bulk form.
Calcium hydroxide can also be dehydrated to calcium oxide by heating it at 580 °C for a few hours. This reaction is generally preferred, as it takes place at lower temperatures, which are much easier to attain. As mentioned before, powdered calcium hydroxide does not convert as good as bulk form.
In both cases, after the reaction has completed, the hot quicklime should be left in a container filled with a neutral gas, such as nitrogen, to prevent the oxide from reacting with water vapors or carbon dioxide. Oxygen can also be used. If synthesized in large amounts, this is not required, as only the surface oxide will convert to limestone, which acts as a protective layer for the inner quicklime.
If the temperature is high enough, the calcium oxide will harden into fragile chunks, which can be easily separated from the leftover calcium carbonate/hydroxide by carefully sifting it through a sieve.
Projects
- Homemade cement/concrete
- Drying solvents
- Desiccator
- Make calcium salts
- Make calcium carbonate
- Make calcium cyanamide
Handling
Safety
Calcium oxide is extremely corrosive to the human tissue as it reacts vigorously with the water from tissues, releasing lots of heat, which causes severe burns. Quicklime is not normally a fire hazard, but sometimes its reaction with water can release sufficient heat to ignite combustible materials. Neutralizing calcium oxide even with a weak acid will release copious amounts of heat. Intentionally mixing calcium oxide with water in a controlled and well-thought out setup can be used to produce the somewhat more stable calcium hydroxide. Gloves, face mask and goggles should be worn when handling the powder, as most the commercial powder is extremely fine and can easily become airborne.
Storage
Calcium oxide should be stored in closed or sealed bottles, in a dry place, to prevent it from absorbing water and carbon dioxide from air. Small amounts of CaO will rapidly degrade in contact with air, while larger amounts take much longer.
Disposal
Calcium oxide should be neutralized before being discarded of. It doesn't pose any hazards to the environment after being neutralized and can be safely dumped in the ground, preferably construction sites. Avoid dumping it on grassland as it will burn it. Dumping it in trash is also an alternative.