Difference between revisions of "Hydrogen cyanide"
Diachrynic (Talk | contribs) |
|||
| Line 7: | Line 7: | ||
| OtherNames = Carbon hydride nitride<br>Formic anammonide<br>Hydrocyanic acid<br>Methanenitrile<br>Prussic acid | | OtherNames = Carbon hydride nitride<br>Formic anammonide<br>Hydrocyanic acid<br>Methanenitrile<br>Prussic acid | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = HCN_Pok.jpg |
| − | | ImageSize = | + | | ImageSize = 300px |
| − | | ImageAlt = | + | | ImageCaption = Liquid HCN by Pok |
| + | | ImageAlt = Ampoule of liquid HCN | ||
| ImageName = | | ImageName = | ||
| ImageFile1 = | | ImageFile1 = | ||
| Line 197: | Line 198: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=9543 Distilling HCN from potassium ferrocyanide...] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=9543 Distilling HCN from potassium ferrocyanide...] | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=23590 What does HCN or H2S poisoning feels like?] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=23590 What does HCN or H2S poisoning feels like?] | ||
| + | *[https://www.sciencemadness.org/whisper/viewthread.php?tid=160124 Preparation of Hydrogen Cyanide] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Latest revision as of 12:40, 26 January 2024
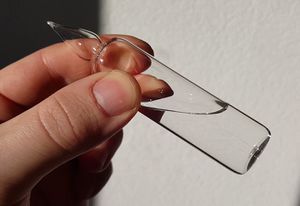 Liquid HCN by Pok
| |
| Names | |
|---|---|
| IUPAC names
Formonitrile (substitutive)
Hydridonitridocarbon (additive) | |
| Other names
Carbon hydride nitride
Formic anammonide Hydrocyanic acid Methanenitrile Prussic acid | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| HCN | |
| Molar mass | 27.0253 g/mol |
| Appearance | Colorless volatile liquid |
| Odor | Bitter almond-like |
| Density | 0.687 g/cm3 |
| Melting point | 13.4 °C (56.1 °F; 286.5 K) |
| Boiling point | 25.6 °C (78.1 °F; 298.8 K) |
| Miscible | |
| Solubility | Reacts with amines, hydrogen peroxide Miscible with ethanol Slightly soluble in diethyl ether |
| Vapor pressure | 630 mmHg (at 20 °C) |
| Acidity (pKa) | 9.21 |
| Thermochemistry | |
| Std molar
entropy (S |
113.01 J·K−1·mol−1 |
| Std enthalpy of
formation (ΔfH |
109.9 kJ·mol−1 |
| Hazards | |
| Safety data sheet | Matheson |
| Flash point | −17.8 °C |
| Related compounds | |
| Related compounds
|
Acetonitrile Cyanogen |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrogen cyanide, also known as prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils just above room temperature, at 25.6 °C.
The exact classification of HCN as organic or inorganic remains unresolved. Going by the C-H bond definition, HCN would be organic, but in aqueous solution it dissociates into H+ and CN-, and since the cyanide ion is a pseudohalogen, this makes it inorganic. Hydrogen cyanide is considered a borderline chemical compound.
Contents
Properties
Chemical
Hydrogen cyanide reacts with sodium hydroxide to yield sodium cyanide.
- HCN + NaOH → NaCN + H2O
Hydrogen cyanide produces the nucleophilic cyanide ion CN, which can attack the carbonyl carbon of an aldehyde or ketone, forming a hydroxyl nitrile or cyanohydrin.
- R-CH=O + HCN → R-HC(OH)-CN
Physical
Hydrogen cyanide is a highly toxic colorless liquid with a bitter almond smell. It is miscible with water and ethanol, and slightly soluble in diethyl ether. HCN melts at -13.4 °C and boils at 25.6 °C. Its density is 0.687 g/cm3.
When hydrogen cyanide is frozen using liquid nitrogen, luminous sparks appear as it freezes. This is caused by a pyroeletric effect, probably induced by the phase transition from a centrosymmetric to a non-centrosymmetric space group.[1]
Availability
Anhydrous hydrogen cyanide is only available to large chemical entities and cannot be purchased by individuals or small companies.
HCN is is listed on schedule 3 of the Chemical Weapons Convention, meaning all transactions are monitored.
HCN absorbed by diatomaceous earth is somewhat easier to come by: it is sometimes sold as an industrial-strength pesticide. The most well known brand is Uragan D2 (Czech Republic). Another, very infamous brand under which this product was sold was Zyklon-B (the agent used to kill people in Nazi gas chambers).
Preparation
Due to its extreme toxicity, HCN production is generally avoided and instead sodium cyanide or other cyanide salts are used for reactions where a cyanide group is required. The procedures below should only performed if you have proper installations and experience.
Adding any non-oxidizing strong acid to sodium or potassium cyanide will liberate hydrogen cyanide, which will rapidly evaporate due to its low boiling point.
- 2 NaCN + H2SO4 → 2 HCN + Na2SO4
- 2 KCN + H2SO4 → 2 HCN + K2SO4
Adding a dilute acid to Prussian blue and heating the flask will also yield hydrogen cyanide gas. However, the yield is variable, generally low, dependent on the acid and concentration used, as well as how the prussian blue was manufactured. The initial reaction is believed to be:[2]
- Fe4[Fe(CN)6]3 + 24 HCl → 4 H3[FeCl6] + 3 H4[Fe(CN)6]
The formed H4[Fe(CN)6] will probably decompose further to yield some HCN, potentially in a similar way to the decomposition of the potassium salt with acid, which is stated further down in this section.
The thermal decomposition of amides in the presence of a catalyst will yield hydrogen cyanide gas. An approximate reaction is given below:
- 2 R-CONH2 → 2 HCN + H2O + R-R[Citation needed]
A closely related method is the removal of water from formamide, which can be done in a number of ways. Distillation with anhydrous phosphoric acid, as well as passing it over activated alumina at 250-300 °C, as well as passing it over various metal catalysts such as brass, iron or copper-alloys under mild vacuum (in the range 50-150 Torr) and at temperatures of 400-500 °C, sometimes in the presence of ammonia gas, gives HCN in high yields.[3]
- HCONH2 → HCN + H2O
Moderate heating of potassium ferrocyanide, K4[Fe(CN)6], and ~35% H2SO4 will produce produce HCN gas which may be dried with calcium chloride.[4] The reaction is:[2]
- 2 K4[Fe(CN)6] + 6 H2SO4 → 6 KHSO4 + K2Fe[Fe(CN)6] + 6 HCN
HCN may be prepared quantitatively without the use of acids from Hg(CN)2 by treatment with aluminium metal in water:[5][3]
- Hg(CN)2 + Al + 3 H2O → 2 HCN + Al(OH)3 + ½ H2 + Hg
Very pure HCN may be obtained by the dehydration of ammonium formate with phosphorus pentoxide in a molar ratio of 1:2.5 with heating.[3][6]
- 3 HCOONH4 + P2O5 → 3 HCN + 2 H3PO4
A crude method of obtaining hydrogen cyanide involves adding acid to ground cherry, bitter almond, peach or apricot seed kernels. The acid breaks down the amygdalin releasing HCN gas.
The combustion of nitrile containing plastic materials will give off very impure hydrogen cyanide gas.
Projects
Seriously, if you really want to make experiments with cyanides, it's MUCH safer to use cyanide salts. Experimenting with hydrogen cyanide is not worth the risk.
Handling
Safety
Hydrogen cyanide is lethal. Air concentrations of 100–200 ppm in air will kill a human within 10-60 minutes, while 2000 ppm (about 2380 mg/m3) will kill a human in about 1 minute. Since it boils slightly above room temperature, open containers are a big no-no, and metal cylinders are instead employed, which severely limit its evaporation.
Contact of HCN with bases and amines may cause violent polymerization and even explosion.
Hydrogen cyanide will also attack certain plastic and rubber materials.
In case of poisoning, glucose, methylene blue or sodium thiosulfate can be used as antidotes.
Storage
DO NOT STORE HCN! EVER! If you absolutely want to, you can make your own Uragan D2 and store that.
Disposal
Hydrogen cyanide can be neutralized with a variety of chemicals, such as hydrogen peroxide, sodium hypochlorite (bleach), which will convert it to the less toxic isocyanate species. Sodium thiosulfate will convert it to thiocyanate, which is less toxic. These two products can be further oxidized to nitrogen, carbon dioxide and water with a strong oxidizer.
References
- ↑ https://www.youtube.com/watch?v=MghUSkNKjYc
- ↑ 2.0 2.1 Gmelins Handbuch der anorganischen Chemie, Eisen Teil B, Verlag Chemie GmbH, Berlin, 8th edition 1932, p. 659, 687
- ↑ 3.0 3.1 3.2 Gmelins Handbuch der anorganischen Chemie, Kohlenstoff Teil D1, Verlag Chemie GmbH, Weinheim/Bergstraße, 8th edition 1971, p. 156, 159
- ↑ L. Gattermann, Justus Liebigs Ann. Chem. 357, 318-319 (1907). https://doi.org/10.1002/jlac.19073570209
- ↑ G. Denigès, Bull. Trav. Soc. Pharm. Bordeux 85, 36 (1947)
- ↑ M. Lebl, Chemicke Listy 64, 877-879 (1970)
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Inorganic compounds
- Organic compounds
- Hydrogen compounds
- Cyanides
- Acids
- Weak acids
- Materials unstable in basic solution
- Things that can kill you very quickly
- Things that should NOT be messed with except by professionals
- Blood agents
- Schedule 3 chemicals