Difference between revisions of "Methyl cellosolve"
From Sciencemadness Wiki
m |
|||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Stub}} | {{Stub}} | ||
| − | |||
| − | |||
{{Chembox | {{Chembox | ||
| − | | Name = | + | | Name = 2-Methoxyethanol |
| Reference = | | Reference = | ||
| IUPACName = 2-Methoxyethanol | | IUPACName = 2-Methoxyethanol | ||
| PIN = | | PIN = | ||
| − | | SystematicName = | + | | SystematicName = 2-Methoxyethanol |
| − | | OtherNames = | + | | OtherNames = Ethylene glycol monomethyl ether<br>EGME<br>Methyl cellosolve |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = 2-methoxyethanol.png |
| ImageSize = | | ImageSize = | ||
| ImageAlt = | | ImageAlt = | ||
| Line 51: | Line 45: | ||
| Abbreviations = | | Abbreviations = | ||
| SMILES = OCCOC | | SMILES = OCCOC | ||
| + | | CASNo = 109-86-4 | ||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| AtmosphericOHRateConstant = | | AtmosphericOHRateConstant = | ||
| − | | Appearance = | + | | Appearance = Colorless liquid |
| BoilingPt = | | BoilingPt = | ||
| − | | BoilingPtC = | + | | BoilingPtC = 124 to 125 |
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| − | | Density = | + | | Density = 0.965 g/cm<sup>3</sup> |
| − | | Formula = | + | | Formula = '''[[Carbon|C]]'''<sub>3</sub>'''[[Hydrogen|H]]'''<sub>8</sub>'''[[Oxygen|O]]'''<sub>2</sub> |
| HenryConstant = | | HenryConstant = | ||
| LogP = | | LogP = | ||
| − | | MolarMass = | + | | MolarMass = 76.09 g/mol |
| MeltingPt = | | MeltingPt = | ||
| − | | MeltingPtC = | + | | MeltingPtC = −85 |
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Ethereal | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | + | | Solubility = Miscible | |
| − | | Solubility = | + | | SolubleOther = Miscible with alcohols |
| − | | SolubleOther = | + | |
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = 6 mmHg (20 °C) |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 96: | Line 91: | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| AutoignitionPt = | | AutoignitionPt = | ||
| − | | ExploLimits = | + | | ExploLimits = 1.8%-14% |
| − | | ExternalMSDS = | + | | ExternalMSDS = [https://www.docdroid.net/Avbto4j/2-methoxyethanol-sa.pdf Sigma-Aldrich] |
| − | | FlashPt = | + | | FlashPt = 39 °C (102 °F; 312 K) |
| LD50 = | | LD50 = | ||
| LC50 = | | LC50 = | ||
| − | | MainHazards = | + | | MainHazards = Irritant |
| NFPA-F = | | NFPA-F = | ||
| NFPA-H = | | NFPA-H = | ||
| Line 112: | Line 107: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = | + | | OtherCompounds = [[Ethylene glycol]] |
}} | }} | ||
}} | }} | ||
| − | + | '''2-Methoxyethanol''', also known as '''ethylene glycol monomethyl ether''' or '''methyl cellosolve''', is a common solvent. | |
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | + | Methyl cellosolve is both a [[primary alcohol]] and an [[ether]]. | |
===Physical=== | ===Physical=== | ||
| − | + | Methyl cellosolve is a colorless liquid, with an ethereal odor. It is miscible with [[water]] and most organic solvents. | |
==Availability== | ==Availability== | ||
| − | + | Many OTC paint and dye solvents have this compound in their composition. Fractional distillation is required to isolate the compound. | |
==Preparation== | ==Preparation== | ||
| − | + | Industrially, methyl cellosolve is produced by the reaction of [[methanol]] with [[ethylene oxide]]. | |
==Projects== | ==Projects== | ||
| − | + | *Paint stripper | |
==Handling== | ==Handling== | ||
| − | |||
===Safety=== | ===Safety=== | ||
| + | Methyl cellosolve is an endocrine disruptor. It is toxic to the bone marrow and testes. | ||
===Storage=== | ===Storage=== | ||
| + | 2-Methoxyethanol should be kept in closed bottles. BHT is sometimes added as stabilizer.<ref>https://www.sigmaaldrich.com/catalog/product/sigald/360503</ref> | ||
===Disposal=== | ===Disposal=== | ||
| + | Incineration outside should suffice. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=1661 Synthesis of symmetrical polyethers, how is it done?] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Organic compounds]] | ||
| + | [[Category:Alcohols]] | ||
| + | [[Category:Ethers]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 22:40, 20 January 2020
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
| |
| Names | |
|---|---|
| IUPAC name
2-Methoxyethanol
| |
| Systematic IUPAC name
2-Methoxyethanol | |
| Other names
Ethylene glycol monomethyl ether
EGME Methyl cellosolve | |
| Identifiers | |
| 109-86-4 | |
| Jmol-3D images | Image |
| |
| Properties | |
| C3H8O2 | |
| Molar mass | 76.09 g/mol |
| Appearance | Colorless liquid |
| Odor | Ethereal |
| Density | 0.965 g/cm3 |
| Melting point | −85 °C (−121 °F; 188 K) |
| Boiling point | 124 to 125 °C (255 to 257 °F; 397 to 398 K) |
| Miscible | |
| Solubility | Miscible with alcohols |
| Vapor pressure | 6 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 39 °C (102 °F; 312 K) |
| Related compounds | |
| Related compounds
|
Ethylene glycol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
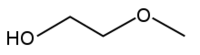
2-Methoxyethanol, also known as ethylene glycol monomethyl ether or methyl cellosolve, is a common solvent.
Contents
Properties
Chemical
Methyl cellosolve is both a primary alcohol and an ether.
Physical
Methyl cellosolve is a colorless liquid, with an ethereal odor. It is miscible with water and most organic solvents.
Availability
Many OTC paint and dye solvents have this compound in their composition. Fractional distillation is required to isolate the compound.
Preparation
Industrially, methyl cellosolve is produced by the reaction of methanol with ethylene oxide.
Projects
- Paint stripper
Handling
Safety
Methyl cellosolve is an endocrine disruptor. It is toxic to the bone marrow and testes.
Storage
2-Methoxyethanol should be kept in closed bottles. BHT is sometimes added as stabilizer.[1]
Disposal
Incineration outside should suffice.
References
Relevant Sciencemadness threads
Categories:
- Article stubs
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Alcohols
- Ethers
- Liquids