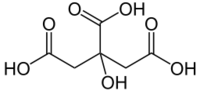
Citric acid
 Food grade citric acid.
| |
| |
| Names | |
|---|---|
| IUPAC name
3-carboxy-3-hydroxypentane-1,5-dioic acid
| |
| Systematic IUPAC name
Citric acid | |
| Other names
3-carboxy-3-hydroxypentanedioic acid
2-hydroxy-1,2,3-propanetricarboxylic acid | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C6H8O7 | |
| Molar mass | 192.12 g/mol |
| Appearance | slightly hygroscopic colorless solid |
| Odor | Odorless |
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (monohydrate, at 18 °C) |
| Melting point | 156 °C (313 °F; 429 K) (decomposes) |
| Boiling point | 310 °C (590 °F; 583 K) (decomposes from 175 °C) |
| 117.43 g/100 mL (10 °C) 147.76 g/100 mL (20 °C) 180.89 g/100 mL (30 °C) 220.19 g/100 mL (40 °C) 382.48 g/100 mL (80 °C) 547.79 g/100 mL (100 °C) | |
| Solubility | Soluble in ethanol, diethyl ether, ethyl acetate, DMSO, methanol Insoluble in benzene, carbon disulfide, chloroform, toluene, xylene |
| Solubility in ethanol | 62 g/100 g (25 °C) |
| Solubility in amyl acetate | 4.41 g/100 g (25 °C) |
| Solubility in diethyl ether | 1.05 g/100 g (25 °C) |
| Solubility in 1,4-Dioxane | 35.9 g/100 g (25 °C) |
| Acidity (pKa) | pKa1 = 3.13 pKa2 = 4.76 pKa3 = 6.39, 6.40 |
| Viscosity | 6.5 cP (50% aq. sol.) |
| Thermochemistry | |
| Std molar
entropy (S |
252.1 J/(mol·K) |
| Std enthalpy of
formation (ΔfH |
-1548.8 kJ/mol |
| Hazards | |
| Safety data sheet | ScienceLab |
| Flash point | 155 °C (311 °F; 428 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
3000 mg/kg (rats, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Citric acid or 2-hydroxy-1,2,3-propanetricarboxylic acid (IUPAC name: 2-hydroxypropane-1,2,3-tricarboxylic acid) is a weak organic acid, mostly used in the food industry, where it serves as a preservative and a food additive that gives a pleasant sour taste. It has the chemical formula C6H8O7.
Contents
Properties
Chemical
Citric acid will react in solution with bases, carbonates and bicarbonates, as well as reactive metals, such as magnesium, to form their respective salts, citrates.
- Mg + C6H8O7 → C6H6O7Mg + H2
As can be seen with its use as a preservative for fruits, it often serves as a reducing agent, similar to ascorbic acid, both in nature and some syntheses.
Citric acid has a hydroxyl group, with which it can form some mineral acid esters. It also may be used in the production of some exotic polyesters.
Physical
Citric acid is at standard conditions a white hygroscopic crystalline powder. It exists either in an anhydrous (water-free) form or as a monohydrate. The monohydrate can be converted to the anhydrous form by heating above 78 °C. It is soluble in water, ethanol, diethyl ether, ethyl acetate, DMSO and insoluble in benzene, toluene, chloroform, carbon disulfide.
Availability
Citric acid is available in stores as lemon salt, either pure or mixed with other additives. It can be found in pickling and canning sections of grocery stores already in pure, food-grade form.
Williams-Sonoma sells citric acid in small glass jars.
Legal
While not controlled, there were some reports in the past years of law enforcement agencies monitoring the purchase of large amounts of citric acid, mainly due to its use in the production of HMTD. Some drug users use citric acid as cutting agent.
Preparation
Citric acid can be prepared by reacting a citrate salt with a stronger acid.
Industrially it is extracted from Aspergillus niger cultures. However, because it's dirt cheap, citric acid is easier to buy than to make it yourself.
Projects
- Alka-Seltzer rocket
- Anthocyanin extraction
- HMTD synthesis
- Propane-1,2,3-tricarboxylic acid synthesis
- Acetonedicarboxylic acid preparation
- Ferric ammonium citrate preparation
- Triethyl citrate synthesis
- Trimethyl citrate synthesis
- Benedict's reagent
- Citrazinic acid synthesis
- Preparation of Nitrocitric acid
Handling
Safety
Being a weak acid, it is not very toxic, but in high concentrations can irritate the skin and sensitive tissues. Ingesting large quantities of citric acid will upset the stomach and cause digestive problems, as well as metabolic acidosis[1].
Storage
Solid citric acid should be stored in closed bottles, in a dry place.
Storage of citrate containing solutions often promotes the growth of bacteria that may metabolize it. Adding an antibacterial can prevents this.
Disposal
Citric acid can be neutralized before disposal, though this is not always necessary. It can be poured down the drain or dumped in the ground.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Acids
- Weak acids
- Carboxylic acids
- Citrates
- Reducing agents
- Readily available chemicals
- Materials available as food grade
- Edible chemicals
- Essential reagents
- Biologically-derived compounds