Difference between revisions of "Picric acid"
| (12 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Chembox | |
| − | [ | + | | Name = Picric acid |
| − | '''Picric acid''' is a bright yellow explosive. It is also known as ''' | + | | Reference = |
| + | | IUPACName = 2,4,6-Trinitrophenol | ||
| + | | PIN = 2,4,6-Trinitrophenol | ||
| + | | SystematicName = | ||
| + | | OtherNames = 2,4,6-TNP<br>2,4,6-Trinitro-1-phenol<br>2-Hydroxy-1,3,5-trinitrobenzene<br>Carbazotic acid<br>Chrysolepic acid<br>Melinite<br>Phenol trinitrate<br>Picronitric acid<br>TNP<br>Trinitrophenol | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = | ||
| + | | ImageFile1 = Picric acid crystals in solution by NeonPulse.jpg | ||
| + | | ImageSize1 = 300 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageCaption1 = Picric acid recrystallized from solution, showing large needles. | ||
| + | | ImageFile2 = Picric acid structure.png | ||
| + | | ImageSize2 = 200 | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Yellow solid | ||
| + | | BoilingPt = > | ||
| + | | BoilingPtC = 300 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = (explodes) | ||
| + | | Density = 1.763 g/cm<sup>3</sup> | ||
| + | | Formula = C<sub>6</sub>H<sub>3</sub>N<sub>3</sub>O<sub>7</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = 1.44 | ||
| + | | MolarMass = 229.10 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 122.5 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| + | | pKa = 0.38 | ||
| + | | pKb = | ||
| + | | Solubility = 1.27 g/100 ml | ||
| + | | SolubleOther = Very soluble in [[acetone]] | ||
| + | | Solubility1 = 10 g/100 g (20 °C) | ||
| + | | Solvent1 = benzene | ||
| + | | Solubility2 = 2.85 g/100 g (20 °C) | ||
| + | | Solvent2 = chloroform | ||
| + | | Solubility3 = 1.53 g/100 g (20 °C) | ||
| + | | Solvent3 = diethyl ether | ||
| + | | Solubility4 = 8.3 g/100 g (20 °C) | ||
| + | | Solvent4 = ethanol | ||
| + | | Taste = Bitter | ||
| + | | VaporPressure = 7.5·10<sup>-7</sup> mmHg (25 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = 2561.48 kJ/mol | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = 7,350 m/s | ||
| + | | REFactor = 1.20 | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 300 °C (explodes) | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.fishersci.com/shop/msdsproxy?productName=A253100&productDescription=PICRIC+ACID+REAGENT+ACS+100G&catNo=A253-100&vendorId=VN00033897&storeId=10652 FisherScientific] (70%) | ||
| + | | FlashPt = 150 °C | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant<br>Harmful<br>Explosive | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Styphnic acid]]<br>[[Trinitrophloroglucinol]]<br>[[Trinitrotoluene]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Picric acid''' is an organic chemical compound, a bright yellow explosive. It is also known as '''trinitrophenol''' or '''TNP''', a name reflecting the chemical it is created from. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Picric acid is an organic acid, and will react with many metals and bases to form salts known as picrates, as well as forming a few complexes. | + | Picric acid is an organic acid, and will react with many metals and bases to form salts known as picrates, which are more sensitive than picric acid itself, as well as forming a few complexes. |
As an acid, contact with metals form picrate salts, nearly of all which are energetic to some degree. Some picrate salts are less sensitive than picric acid while some, such as [[lead picrate]], are more sensitive than the acid. This is one of the biggest concerns with storage; if picric acid is stored incorrectly, for example in contact with a metal lid, the shock sensitive picrate salts could perhaps detonate and initiate the rest of the picric acid. | As an acid, contact with metals form picrate salts, nearly of all which are energetic to some degree. Some picrate salts are less sensitive than picric acid while some, such as [[lead picrate]], are more sensitive than the acid. This is one of the biggest concerns with storage; if picric acid is stored incorrectly, for example in contact with a metal lid, the shock sensitive picrate salts could perhaps detonate and initiate the rest of the picric acid. | ||
| Line 12: | Line 129: | ||
===Physical=== | ===Physical=== | ||
| − | Picric acid is a pale yellow solid that dissolves to form very bright yellow solutions. This yellow | + | Picric acid is a pale yellow solid that dissolves to form very bright yellow solutions. This yellow color is very staining to all surfaces as well as skin, and due to this fact it was historically used as a dye. It should be noted that many plastic surfaces, being organic, will be permanently stained by picric acid if it is left too long. |
Picric acid is mostly insoluble in freezing water, allowing for easy precipitation and recrystallization from boiling water. It is much more soluble in [[ethanol]] or [[methanol]]. | Picric acid is mostly insoluble in freezing water, allowing for easy precipitation and recrystallization from boiling water. It is much more soluble in [[ethanol]] or [[methanol]]. | ||
| − | |||
| − | |||
Picric acid melts at 122 degrees Celsius before igniting and burning in air producing black smoke. It is flammable and will burn well despite a poor [[oxygen balance]]. | Picric acid melts at 122 degrees Celsius before igniting and burning in air producing black smoke. It is flammable and will burn well despite a poor [[oxygen balance]]. | ||
| + | |||
| + | ===Explosive=== | ||
| + | Its explosive properties are similar to [[trinitrotoluene|TNT]], considered to be slightly more powerful and slightly more sensitive than TNT. It is very difficult to detonate on its own via impact or friction, with considerable force needed to purposefully detonate the dry powder even when confined, making accidental detonation a minimal possibility unless highly sensitive picrates are present. | ||
==Availability== | ==Availability== | ||
| Line 26: | Line 144: | ||
==Preparation== | ==Preparation== | ||
| − | Trinitrolphenol can be prepared by first 'sulfonating' phenol by heating it with concentrated [[sulfuric acid]] before the addition of [[nitric acid]] or a [[Potassium nitrate|nitrate salt]]. This is convenient as nitration baths require concentrated sulfuric acid anyway but the sulfonation | + | Trinitrolphenol can be prepared by first 'sulfonating' phenol by heating it with concentrated [[sulfuric acid]] before the addition of [[nitric acid]] or a [[Potassium nitrate|nitrate salt]]. This is convenient as nitration baths require concentrated sulfuric acid anyway but the sulfonation moderates the reaction, allowing for a much better yield. |
Although phenol is not a terribly common lab chemical, both [[Salicylic acid|salicylic]] and [[acetylsalicylic acid]] can be used, the sulfonation process splitting up the molecules producing [[carbon dioxide]], and [[acetic acid]] if acetylsalicylic acid is used. These chemicals are very easy to acquire for the home chemist, increasing the appeal of picric acid.<ref>http://www.sciencemadness.org/talk/files.php?pid=311232&aid=28045</ref> | Although phenol is not a terribly common lab chemical, both [[Salicylic acid|salicylic]] and [[acetylsalicylic acid]] can be used, the sulfonation process splitting up the molecules producing [[carbon dioxide]], and [[acetic acid]] if acetylsalicylic acid is used. These chemicals are very easy to acquire for the home chemist, increasing the appeal of picric acid.<ref>http://www.sciencemadness.org/talk/files.php?pid=311232&aid=28045</ref> | ||
| Line 47: | Line 165: | ||
Picric acid, being an aromatic compound can be destroyed using [[Fenton's reagent]]. The best disposal formula consists of a peroxide solution of at least 25%, at a pH of 2-3. The neutralization process takes several hours and doesn't produce any dangerous side products.<ref>http://www.researchgate.net/publication/231562666_Oxidative_Destruction_of_Picric_Acid_in_Aqueous_Media_by_Fentons_Reagent</ref> | Picric acid, being an aromatic compound can be destroyed using [[Fenton's reagent]]. The best disposal formula consists of a peroxide solution of at least 25%, at a pH of 2-3. The neutralization process takes several hours and doesn't produce any dangerous side products.<ref>http://www.researchgate.net/publication/231562666_Oxidative_Destruction_of_Picric_Acid_in_Aqueous_Media_by_Fentons_Reagent</ref> | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="220" position="center" columns="4" orientation="none"> | ||
| + | Picric acid underwater by Tdep.jpg|Picric acid stored under water, showing its bright yellow color in solution and as crystals | ||
| + | Picric acid needles crystals by Sir Gawain.jpg|Picric Acid Recrystallization | ||
| + | </gallery> | ||
==References== | ==References== | ||
| Line 58: | Line 182: | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
| − | [[Category: | + | [[Category:Nitro compounds]] |
| + | [[Category:Nitroaromatics]] | ||
[[Category:Acids]] | [[Category:Acids]] | ||
[[Category:Phenols]] | [[Category:Phenols]] | ||
| Line 64: | Line 189: | ||
[[Category:Energetic materials]] | [[Category:Energetic materials]] | ||
[[Category:High explosives]] | [[Category:High explosives]] | ||
| + | [[Category:Secondary explosives]] | ||
[[Category:Dyes]] | [[Category:Dyes]] | ||
Latest revision as of 09:31, 23 September 2023
 Picric acid recrystallized from solution, showing large needles.
| |
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-Trinitrophenol
| |
| Preferred IUPAC name
2,4,6-Trinitrophenol | |
| Other names
2,4,6-TNP
2,4,6-Trinitro-1-phenol 2-Hydroxy-1,3,5-trinitrobenzene Carbazotic acid Chrysolepic acid Melinite Phenol trinitrate Picronitric acid TNP Trinitrophenol | |
| Properties | |
| C6H3N3O7 | |
| Molar mass | 229.10 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | 1.763 g/cm3 |
| Melting point | 122.5 °C (252.5 °F; 395.6 K) |
| Boiling point | > 300 °C (572 °F; 573 K) (explodes) |
| 1.27 g/100 ml | |
| Solubility | Very soluble in acetone |
| Solubility in benzene | 10 g/100 g (20 °C) |
| Solubility in chloroform | 2.85 g/100 g (20 °C) |
| Solubility in diethyl ether | 1.53 g/100 g (20 °C) |
| Solubility in ethanol | 8.3 g/100 g (20 °C) |
| Vapor pressure | 7.5·10-7 mmHg (25 °C) |
| Acidity (pKa) | 0.38 |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | FisherScientific (70%) |
| Flash point | 150 °C |
| Related compounds | |
| Related compounds
|
Styphnic acid Trinitrophloroglucinol Trinitrotoluene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
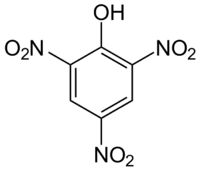
Picric acid is an organic chemical compound, a bright yellow explosive. It is also known as trinitrophenol or TNP, a name reflecting the chemical it is created from.
Contents
Properties
Chemical
Picric acid is an organic acid, and will react with many metals and bases to form salts known as picrates, which are more sensitive than picric acid itself, as well as forming a few complexes.
As an acid, contact with metals form picrate salts, nearly of all which are energetic to some degree. Some picrate salts are less sensitive than picric acid while some, such as lead picrate, are more sensitive than the acid. This is one of the biggest concerns with storage; if picric acid is stored incorrectly, for example in contact with a metal lid, the shock sensitive picrate salts could perhaps detonate and initiate the rest of the picric acid.
Upon reaction with ammonia, ammonium picrate, or Dunnite, a historical military explosive is formed. Reduction with a sulfite produces picramic acid.
Physical
Picric acid is a pale yellow solid that dissolves to form very bright yellow solutions. This yellow color is very staining to all surfaces as well as skin, and due to this fact it was historically used as a dye. It should be noted that many plastic surfaces, being organic, will be permanently stained by picric acid if it is left too long.
Picric acid is mostly insoluble in freezing water, allowing for easy precipitation and recrystallization from boiling water. It is much more soluble in ethanol or methanol.
Picric acid melts at 122 degrees Celsius before igniting and burning in air producing black smoke. It is flammable and will burn well despite a poor oxygen balance.
Explosive
Its explosive properties are similar to TNT, considered to be slightly more powerful and slightly more sensitive than TNT. It is very difficult to detonate on its own via impact or friction, with considerable force needed to purposefully detonate the dry powder even when confined, making accidental detonation a minimal possibility unless highly sensitive picrates are present.
Availability
It was once used as a dye and for a few other novel chemistry reactions so it is sometimes found in old schools or laboratories. It is very rarely used now due to its explosive properties.
Picric acid is stored under water and is not considered an explosion hazard while wet. Old samples may have dried out, or may have come into contact with metal such as a metal lid and this greatly increases the explosion risk.
Preparation
Trinitrolphenol can be prepared by first 'sulfonating' phenol by heating it with concentrated sulfuric acid before the addition of nitric acid or a nitrate salt. This is convenient as nitration baths require concentrated sulfuric acid anyway but the sulfonation moderates the reaction, allowing for a much better yield.
Although phenol is not a terribly common lab chemical, both salicylic and acetylsalicylic acid can be used, the sulfonation process splitting up the molecules producing carbon dioxide, and acetic acid if acetylsalicylic acid is used. These chemicals are very easy to acquire for the home chemist, increasing the appeal of picric acid.[1]
Projects
- Producing picramic acid
- Ammonium picrate synthesis
Handling
Safety
There is always a non negligible chance of an unexpected detonation, and steps should always be taken to minimize this risk. The dangers of static electricity when dealing with explosives should also always be considered. Picric is a secondary explosive however, and requires substantially confinement and energy to detonate.
Picric acid is toxic through skin contact and inhalation. Skin contact will result in bright yellow stains. Inhalation can be noticed in large amounts due to a bitter taste in the mouth, but this should not be used as a detection method but a sign that you are breathing in too much of the toxic acid.
Storage
The sensitivity of picric acid can be dramatically increased by the reaction with other chemicals, especially metals. When stored, it should be stored under water in a glass container, making sure it does not react or dry out unexpectedly.
Disposal
Dry TNP is considered an explosion hazard. When any dry bottles of TNP is found in the public, a bomb squad is usually called to dispose of the bottle, especially if the bottle has a metal cap.
Picric acid, being an aromatic compound can be destroyed using Fenton's reagent. The best disposal formula consists of a peroxide solution of at least 25%, at a pH of 2-3. The neutralization process takes several hours and doesn't produce any dangerous side products.[2]
Gallery
References
- ↑ http://www.sciencemadness.org/talk/files.php?pid=311232&aid=28045
- ↑ http://www.researchgate.net/publication/231562666_Oxidative_Destruction_of_Picric_Acid_in_Aqueous_Media_by_Fentons_Reagent

