Difference between revisions of "Glycerol"
Brain&Force (Talk | contribs) |
|||
| (18 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[ | + | {{Chembox |
| − | '''Glycerol''', alternatively spelled '''glycerin''', or '''glycerine''', is a simple [[sugar alcohol]] sometimes used as a solvent. It is a polyol, consisting of a propane molecule with one hydrogen on each of the carbons being substituted by a [[hydroxyl]] group. Glycerol is sometimes used as a laboratory solvent, though this is made difficult by its high viscosity. It is also used in the manufacture of the well-known explosive [[nitroglycerin]]. | + | | Name = Glycerol |
| + | | Reference = | ||
| + | | IUPACName = Propane-1,2,3-triol | ||
| + | | PIN = Propane-1,2,3-triol | ||
| + | | SystematicName = | ||
| + | | OtherNames = 1,2,3-Propanetriol<br>1,2,3-Trihydroxypropane<br>Glycerin<br>Glycerine<br>Glycyl alcohol<br>Propanetriol | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Glycerol.png | ||
| + | | ImageSize = 250 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless viscous liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 290 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 1.261 g/cm<sup>3</sup> | ||
| + | | Formula = C<sub>3</sub>H<sub>8</sub>O<sub>3</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 92.09 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 17.8 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = Miscible | ||
| + | | SolubleOther = Miscible with [[ethanol]], [[methanol]], [[propylene glycol]]<br>Insoluble in [[acetone]], [[benzene]], [[carbon disulfide]], [[carbon tetrachloride|CCl<sub>4</sub>]], [[chloroform]], [[diethyl ether]], [[petroleum ether]], [[THF]], [[vegetable oil]]s | ||
| + | | Solvent = | ||
| + | | Taste = Sweet | ||
| + | | VaporPressure = 0.003 mmHg (50°C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.docdroid.net/5B7b40s/glycerol-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = 160 °C (320 °F; 433 K) (closed cup)<br>176 °C (349 °F; 449 K) (open cup) | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Ethylene glycol]]<br>[[Propylene glycol]]<br>[[Erythritol]] | ||
| + | }} | ||
| + | }} | ||
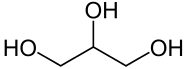
| + | '''Glycerol''', alternatively spelled '''glycerin''', or '''glycerine''', is a simple [[sugar alcohol]] sometimes used as a solvent. It is a polyol, consisting of a propane molecule with one hydrogen on each of the carbons being substituted by a [[hydroxyl]] group. | ||
| + | |||
| + | Glycerol is sometimes used as a laboratory solvent, though this is made difficult by its high viscosity. It is also used in the manufacture of the well-known explosive [[nitroglycerin]]. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| + | Glycerol has several niche uses in home chemistry. A mixture of glycerol and [[oxalic acid]] can be distilled to produce [[formic acid]]. This reaction occurs in several steps, at temperatures above 100 °C. | ||
| − | + | When glycerin is heated to 280 °C, it decomposes into [[acrolein]]: | |
| + | |||
| + | : (CH<sub>2</sub>OH)<sub>2</sub>CHOH → CH<sub>2</sub>=CHCHO + 2 H<sub>2</sub>O | ||
| + | |||
| + | Perhaps most famously, it can be [[nitration|nitrated]] using [[sulfuric acid|sulfuric]] and [[nitric acid]]s in an ice bath to produce [[nitroglycerin]], a sensitive liquid high explosive used to make [[dynamite]]. | ||
| + | |||
| + | It can also be used as an inert solvent for producing extracts from plants or carrying out organic reactions. | ||
===Physical=== | ===Physical=== | ||
| + | Glycerol is a colorless, viscous, and odorless liquid at room temperature with a mild sweet taste similar to artificial sweeteners. It is soluble in water, but has limited solubility in most organic solvents such as [[acetone]], [[chloroform]], and [[diethyl ether]]. It is hygroscopic. | ||
| − | |||
==Availability== | ==Availability== | ||
Glycerol can be found in many pharmacies and grocery stores where it is used as "skin protectant". As with many medical and health products, it is sold at a very high markup, which means that buying glycerol online from certain wholesalers is actually much cheaper. | Glycerol can be found in many pharmacies and grocery stores where it is used as "skin protectant". As with many medical and health products, it is sold at a very high markup, which means that buying glycerol online from certain wholesalers is actually much cheaper. | ||
==Preparation== | ==Preparation== | ||
| − | Glycerol is produced by the hydrolysis, or saponification, of plant and animal fats using a strong base. It is usually simpler to purchase it rather than go through the process of purifying the product from this reaction, though. | + | Glycerol is produced by the hydrolysis, or saponification, of plant and animal fats using a strong base, such as [[sodium hydroxide]]. It is usually simpler to purchase it rather than go through the process of purifying the product from this reaction, though. |
==Projects== | ==Projects== | ||
*Make [[formic acid]] | *Make [[formic acid]] | ||
*Make [[nitroglycerin]] | *Make [[nitroglycerin]] | ||
| + | *Make [[allyl alcohol]] | ||
| + | *Make [[acrolein]] | ||
*Antifreeze | *Antifreeze | ||
| − | = | + | *Make copper-polyol complex<ref>https://www.youtube.com/watch?v=EKj3Oa5GTcM</ref> |
| − | Glycerol is more-or-less nontoxic, as it is an important biological chemical. Food-grade glycerol can be tasted for those that are curious, though external medicinal grades cannot be guaranteed safe for consumption. | + | ==Handling== |
| + | ===Safety=== | ||
| + | Glycerol is more-or-less nontoxic, as it is an important biological chemical. Food-grade glycerol can be tasted for those that are curious, though external medicinal grades cannot be guaranteed safe for consumption (Sciencemadness user Ave369 used medical grade glycerol to soften homemade vodka, and nothing bad happened to her). | ||
| + | |||
| + | ===Storage=== | ||
| + | Glycerol should be stored in sealed bottles, as it is hygroscopic and it is extremely difficult to remove the water. | ||
| + | |||
| + | ===Disposal=== | ||
| + | Glycerol poses little toxicity to the environment and can be safely poured down the drain. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=22180 Drying Glycerol] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=7036 Valorizing the Glycerol from Biodiesel Production] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Organic compounds]] | ||
| + | [[Category:Alcohols]] | ||
| + | [[Category:Polyols]] | ||
| + | [[Category:Sugar alcohols]] | ||
| + | [[Category:Solvents]] | ||
| + | [[Category:Liquids]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Materials available as food grade]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 14:50, 18 November 2023
| |
| Names | |
|---|---|
| IUPAC name
Propane-1,2,3-triol
| |
| Preferred IUPAC name
Propane-1,2,3-triol | |
| Other names
1,2,3-Propanetriol
1,2,3-Trihydroxypropane Glycerin Glycerine Glycyl alcohol Propanetriol | |
| Properties | |
| C3H8O3 | |
| Molar mass | 92.09 g/mol |
| Appearance | Colorless viscous liquid |
| Odor | Odorless |
| Density | 1.261 g/cm3 |
| Melting point | 17.8 °C (64.0 °F; 290.9 K) |
| Boiling point | 290 °C (554 °F; 563 K) |
| Miscible | |
| Solubility | Miscible with ethanol, methanol, propylene glycol Insoluble in acetone, benzene, carbon disulfide, CCl4, chloroform, diethyl ether, petroleum ether, THF, vegetable oils |
| Vapor pressure | 0.003 mmHg (50°C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 160 °C (320 °F; 433 K) (closed cup) 176 °C (349 °F; 449 K) (open cup) |
| Related compounds | |
| Related compounds
|
Ethylene glycol Propylene glycol Erythritol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glycerol, alternatively spelled glycerin, or glycerine, is a simple sugar alcohol sometimes used as a solvent. It is a polyol, consisting of a propane molecule with one hydrogen on each of the carbons being substituted by a hydroxyl group.
Glycerol is sometimes used as a laboratory solvent, though this is made difficult by its high viscosity. It is also used in the manufacture of the well-known explosive nitroglycerin.
Contents
Properties
Chemical
Glycerol has several niche uses in home chemistry. A mixture of glycerol and oxalic acid can be distilled to produce formic acid. This reaction occurs in several steps, at temperatures above 100 °C.
When glycerin is heated to 280 °C, it decomposes into acrolein:
- (CH2OH)2CHOH → CH2=CHCHO + 2 H2O
Perhaps most famously, it can be nitrated using sulfuric and nitric acids in an ice bath to produce nitroglycerin, a sensitive liquid high explosive used to make dynamite.
It can also be used as an inert solvent for producing extracts from plants or carrying out organic reactions.
Physical
Glycerol is a colorless, viscous, and odorless liquid at room temperature with a mild sweet taste similar to artificial sweeteners. It is soluble in water, but has limited solubility in most organic solvents such as acetone, chloroform, and diethyl ether. It is hygroscopic.
Availability
Glycerol can be found in many pharmacies and grocery stores where it is used as "skin protectant". As with many medical and health products, it is sold at a very high markup, which means that buying glycerol online from certain wholesalers is actually much cheaper.
Preparation
Glycerol is produced by the hydrolysis, or saponification, of plant and animal fats using a strong base, such as sodium hydroxide. It is usually simpler to purchase it rather than go through the process of purifying the product from this reaction, though.
Projects
- Make formic acid
- Make nitroglycerin
- Make allyl alcohol
- Make acrolein
- Antifreeze
- Make copper-polyol complex[1]
Handling
Safety
Glycerol is more-or-less nontoxic, as it is an important biological chemical. Food-grade glycerol can be tasted for those that are curious, though external medicinal grades cannot be guaranteed safe for consumption (Sciencemadness user Ave369 used medical grade glycerol to soften homemade vodka, and nothing bad happened to her).
Storage
Glycerol should be stored in sealed bottles, as it is hygroscopic and it is extremely difficult to remove the water.
Disposal
Glycerol poses little toxicity to the environment and can be safely poured down the drain.