Difference between revisions of "Phthalic anhydride"
(→Relevant Sciencemadness threads) |
(Fixed grammar and spelling, added SMILES code and added structural formula image.) |
||
| (10 intermediate revisions by 3 users not shown) | |||
| Line 7: | Line 7: | ||
| OtherNames = 1,3-Dioxophthalan<br>1,3-Isobenzofurandione<br>1,3-Phthalandione<br>Isobenzofuran-1,3-dione<br>Phthalic anhydride | | OtherNames = 1,3-Dioxophthalan<br>1,3-Isobenzofurandione<br>1,3-Phthalandione<br>Isobenzofuran-1,3-dione<br>Phthalic anhydride | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Phthalic anhydride purified by anewsoul.jpg |
| − | | ImageSize = | + | | ImageSize = 280 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageCaption = Fluffy phthalic anhydride |
| + | | ImageFile1 = Phthalic anhydride structural formula.png | ||
| ImageSize1 = | | ImageSize1 = | ||
| ImageAlt1 = | | ImageAlt1 = | ||
| Line 43: | Line 44: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
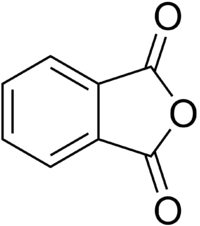
| − | | SMILES = | + | | SMILES = c1ccc2c(c1)C(=O)OC2=O |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 65: | Line 66: | ||
| pKb = | | pKb = | ||
| Solubility = 0.62 g/100 g (20 °C)<br>19.0 g/100 g (100 °C)<br>Slow hydrolysis | | Solubility = 0.62 g/100 g (20 °C)<br>19.0 g/100 g (100 °C)<br>Slow hydrolysis | ||
| − | | SolubleOther = Dissolves and slowly reacts with alcohols<br>Slightly soluble in [[carbon disulfide]] | + | | SolubleOther = Dissolves and slowly reacts with alcohols, amines<br>Soluble in [[acetone]], [[benzene]]<br>Slightly soluble in [[carbon disulfide]], [[diethyl ether]] |
| Solvent = | | Solvent = | ||
| VaporPressure = 0.0015 mmHg (20 °C) | | VaporPressure = 0.0015 mmHg (20 °C) | ||
| Line 88: | Line 89: | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = 570 °C | + | | AutoignitionPt = 570 °C (1058 °F; 843 K) |
| ExploLimits = 1.7%-10.5% | | ExploLimits = 1.7%-10.5% | ||
| ExternalMSDS = [https://www.docdroid.net/4raxOJA/phthalic-anhydride-sa.pdf.html Sigma-Aldrich] | | ExternalMSDS = [https://www.docdroid.net/4raxOJA/phthalic-anhydride-sa.pdf.html Sigma-Aldrich] | ||
| − | | FlashPt = 152 °C | + | | FlashPt = 152 °C (305.6 °F; 425 K) |
| − | | LD50 = | + | | LD50 = 4,020 mg/kg (oral, rat)<br>1,520 mg/kg (oral, mouse)<br>800 mg/kg (oral, cat)<br>800-1,600 mg/kg (oral, rat)<br>2,210 mg/kg (oral, mouse) |
| LC50 = | | LC50 = | ||
| MainHazards = Irritant | | MainHazards = Irritant | ||
| Line 108: | Line 109: | ||
}} | }} | ||
}} | }} | ||
| − | '''Phthalic anhydride''' is an organic compound, the anhydride of phthalic acid. It is an important industrial chemical used in the production of plasticizers. | + | '''Phthalic anhydride''' is an organic compound, the anhydride of phthalic acid. It is an important industrial chemical used in the production of plasticizers, dyestuffs, and pharmaceuticals. |
==Properties== | ==Properties== | ||
| Line 121: | Line 122: | ||
==Preparation== | ==Preparation== | ||
| − | Phthalic anhydride can be prepared from diethylhexyl phthalate, which can be easily found in vinyl gloves, available in most pharmacies and medical supply stores. Look on the box label for gloves that are made with PVC. Grind about 50 g of gloves to flakes in a beaker. Add [[isopropanol]] and reflux the mixture for an hour to extract the diethylhexyl phthalate. Filter the resulting alcoholic solution and then boil off half of its volume. A solution of 10 g of sodium hydroxide is then added to the alcoholic solution of diethylhexyl phthalate and reflux the mixture for another hour, to hydrolyze it to disodium phthalate. Turn off the heating and let it cool. The reaction mixture will separate into two layers, the upper layer is the alcoholic layer, while the bottom is the aqueous layer, containing the disodium | + | Phthalic anhydride can be prepared from diethylhexyl phthalate, which can be easily found in vinyl gloves, available in most pharmacies and medical supply stores. Look on the box label for gloves that are made with PVC. Grind about 50 g of gloves to flakes in a beaker. Add [[isopropanol]] and reflux the mixture for an hour to extract the diethylhexyl phthalate. Filter the resulting alcoholic solution and then boil off half of its volume. A solution of 10 g of sodium hydroxide is then added to the alcoholic solution of diethylhexyl phthalate and reflux the mixture for another hour, to hydrolyze it to disodium phthalate. Turn off the heating and let it cool. The reaction mixture will separate into two layers, the upper layer is the alcoholic layer, while the bottom is the aqueous layer, containing the disodium phthalate. Remove the alcohol layer and collect the disodium phthalate aqueous solution. To the aqueous solution, add 25 ml of fuming hydrochloric acid (12 M) to convert it to phthalic acid. Cool the solution to precipitate the phthalic acid and decant off the water. Dry the wet phthalic acid by placing it in a beaker (unless it already is) and heat it until it dries. Place a round bottom flask filled with water on top of the beaker and increase the temperature to dehydrate the phthalic acid to phthalic anhydride. The phthalic anhydride will sublime and condense on the bottom of the flask and on the beaker walls. The crystals appear as cotton candy. When a sufficient amount of the anhydride has deposited, take the beaker off the hotplate, let it cool, and collect the crystals. Do not wait too much, as the anhydride crystals will get too heavy and will fall back in the phthalic acid melt. Repeat until no more phthalic anhydride condenses.<ref>https://www.youtube.com/watch?v=58Ve69s0qD0</ref> |
| − | Another route involves the oxidation of [[naphthalene]] with [[nitric acid]] or [[vanadium pentoxide]]. [[Mercury(II) sulfate]] and [[sulfuric acid]] can also be used. | + | Another route involves the oxidation of [[naphthalene]] with [[nitric acid]] or [[vanadium pentoxide]]. [[Mercury(II) sulfate]] and [[sulfuric acid]] can also be used. |
| + | |||
| + | The modern industrial route is the vapor-phase oxidation of ortho-[[xylene]] by air over a catalyst. | ||
==Projects== | ==Projects== | ||
| − | *Make luminol | + | * Make luminol |
| − | *Make dyes | + | * Make dyes, for example: |
| − | *Make dimethyl phthalate | + | ** [[Phenolphthalein]] |
| − | *Make phthalimide | + | ** [[Fluorescein]] |
| − | *Make cellulose acetate phthalate | + | ** [[Quinizarin]] |
| + | ** [[Phthalocyanines]] | ||
| + | * Make dimethyl phthalate | ||
| + | * Make [[phthalimide]] | ||
| + | * Make cellulose acetate phthalate | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Phthalic anhydride is | + | Phthalic anhydride is a skin irritant. Phthalates are endocrine disruptors. Avoid breathing dust and vapors of phthalic anhydride. |
===Storage=== | ===Storage=== | ||
| Line 143: | Line 150: | ||
Complete destruction can be achieved with a strong oxidizing solution, such as [[chromic acid]], [[Fenton's reagent]], [[piranha solution]]. | Complete destruction can be achieved with a strong oxidizing solution, such as [[chromic acid]], [[Fenton's reagent]], [[piranha solution]]. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="200" position="center" columns="2" orientation="none"> | ||
| + | Phthalic_acid.jpg|Phthalic acid | ||
| + | Phthalic_anhydride.jpg|Phthalic anhydride (sublimated) | ||
| + | </gallery> | ||
==References== | ==References== | ||
Latest revision as of 13:19, 29 August 2020
 Fluffy phthalic anhydride
| |
| |
| Names | |
|---|---|
| IUPAC name
2-Benzofuran-1,3-dione
| |
| Other names
1,3-Dioxophthalan
1,3-Isobenzofurandione 1,3-Phthalandione Isobenzofuran-1,3-dione Phthalic anhydride | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C8H4O3 | |
| Molar mass | 148.1 g/mol |
| Appearance | White solid |
| Odor | Acrid |
| Density | 1.53 g/cm3 (20 °C) 1.2 g/cm3 (135 °C) |
| Melting point | 131.6 °C (268.9 °F; 404.8 K) |
| Boiling point | 295 °C (563 °F; 568 K) |
| 0.62 g/100 g (20 °C) 19.0 g/100 g (100 °C) Slow hydrolysis | |
| Solubility | Dissolves and slowly reacts with alcohols, amines Soluble in acetone, benzene Slightly soluble in carbon disulfide, diethyl ether |
| Vapor pressure | 0.0015 mmHg (20 °C) |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 152 °C (305.6 °F; 425 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
4,020 mg/kg (oral, rat) 1,520 mg/kg (oral, mouse) 800 mg/kg (oral, cat) 800-1,600 mg/kg (oral, rat) 2,210 mg/kg (oral, mouse) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phthalic anhydride is an organic compound, the anhydride of phthalic acid. It is an important industrial chemical used in the production of plasticizers, dyestuffs, and pharmaceuticals.
Contents
Properties
Chemical
Phthalic anhydride hydrolyzes in water to phthalic acid.
Physical
Phthalic anhydride is a white solid, which slowly reacts with water.
Availability
Phthalic anhydride is sold by various chemical suppliers and it's quite cheap. It can also be purchased online.
Preparation
Phthalic anhydride can be prepared from diethylhexyl phthalate, which can be easily found in vinyl gloves, available in most pharmacies and medical supply stores. Look on the box label for gloves that are made with PVC. Grind about 50 g of gloves to flakes in a beaker. Add isopropanol and reflux the mixture for an hour to extract the diethylhexyl phthalate. Filter the resulting alcoholic solution and then boil off half of its volume. A solution of 10 g of sodium hydroxide is then added to the alcoholic solution of diethylhexyl phthalate and reflux the mixture for another hour, to hydrolyze it to disodium phthalate. Turn off the heating and let it cool. The reaction mixture will separate into two layers, the upper layer is the alcoholic layer, while the bottom is the aqueous layer, containing the disodium phthalate. Remove the alcohol layer and collect the disodium phthalate aqueous solution. To the aqueous solution, add 25 ml of fuming hydrochloric acid (12 M) to convert it to phthalic acid. Cool the solution to precipitate the phthalic acid and decant off the water. Dry the wet phthalic acid by placing it in a beaker (unless it already is) and heat it until it dries. Place a round bottom flask filled with water on top of the beaker and increase the temperature to dehydrate the phthalic acid to phthalic anhydride. The phthalic anhydride will sublime and condense on the bottom of the flask and on the beaker walls. The crystals appear as cotton candy. When a sufficient amount of the anhydride has deposited, take the beaker off the hotplate, let it cool, and collect the crystals. Do not wait too much, as the anhydride crystals will get too heavy and will fall back in the phthalic acid melt. Repeat until no more phthalic anhydride condenses.[1]
Another route involves the oxidation of naphthalene with nitric acid or vanadium pentoxide. Mercury(II) sulfate and sulfuric acid can also be used.
The modern industrial route is the vapor-phase oxidation of ortho-xylene by air over a catalyst.
Projects
- Make luminol
- Make dyes, for example:
- Make dimethyl phthalate
- Make phthalimide
- Make cellulose acetate phthalate
Handling
Safety
Phthalic anhydride is a skin irritant. Phthalates are endocrine disruptors. Avoid breathing dust and vapors of phthalic anhydride.
Storage
Phthalic anhydride should be stored in dry conditions, such as air-tight containers.
Disposal
Can be neutralized with an aqueous alkaline solution to disodium phthalate.
Complete destruction can be achieved with a strong oxidizing solution, such as chromic acid, Fenton's reagent, piranha solution.
Gallery
References
Relevant Sciencemadness threads
- Phthalic Anhydride
- phthalic acid -> phthalic anhydride
- Get phthalic anhydride from phthalic acid
- Need help with prep. of phthalic anhydride from naphthalene
- Conversion of Phthalic Acid to Phthalic Anhydride
- Solubility of phthalic anhydride and resorcinol in sulfuric acid?
- Phthalic anhydride, anyone?
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Carboxylic acid anhydrides
- Phthalates
- Materials that react with water
- Materials unstable in basic solution
- Essential reagents
- Solids

