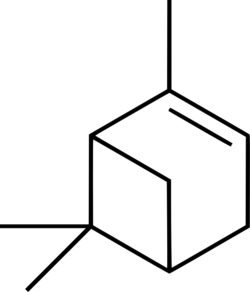
Alpha-pinene
| |
| Names | |
|---|---|
| IUPAC name
(1S,5S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene ((−)-α-Pinene)
| |
| Other names
α-Pinene
Pinene | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Colorless liquid |
| Odor | Pine, turpentine-like |
| Density | 0.858 g/cm3 (at 20 °C) |
| Melting point | −64 °C (−83 °F; 209 K) |
| Boiling point | 155 °C (311 °F; 428 K) |
| 0.000249 g/100 ml (25 °C) | |
| Solubility | Miscible with glacial acetic acid, acetone, chloroform, diethyl ether, ethanol, isopropanol Almost insoluble in glycerol, propylene glycol |
| Vapor pressure | 4.75 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 33 °C (91 °F; 306 K) |
| Related compounds | |
| Related compounds
|
Limonene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-pinene(also written alpha-pinene) is an organic compound with chemical formula C10H16 belonging to the terpenes, a group of biologically important hydrocarbons. It is the most commonly encountered and well researched of the two pinene isomers, and has a number of niche uses in organic synthesis.
Contents
Properties
Chemical
α-pinene and related compounds are commonly utilized in the fragrance industry, and as such, it is a precursor to many of these compounds. Hydration to α-terpineol can be accomplished by the reflux of α-pinene with aqueous sulfuric acid and acetone for a few hours, or by the action of concentrated sulfuric acid in ethanol]. The ester α-terpinyl acetate can be produced by esterification with glacial acetic acid. It can also be rearranged into camphene by strong acid catalysis in glacial acetic acid as a step in the production of camphor. With dilute acids, terpin hydrate becomes the major product.
Addition of iodine or phosphorus trichloride causes aromatisation, leading to p-cymene.
Physical
α-pinene is a lightweight, clear, colorless liquid in pure form, nearly insoluble in water but miscible with organic solvents. The odor of commercial α-pinene depends on its source; as it can be separated from turpentine by distillation, the odor often matches this. α-pinene boils at 155 °C (311 °F) and is only mildly flammable.
Availability
α-pinene is readily available as one of the most major constituents of turpentine and can be distilled in reasonably pure form from it. Turpentine is available as a solvent and paint stripper at many hardware and department stores.
Preparation
α-pinene is more likely to be extracted from turpentine rather than synthesized in the home lab.
Projects
- Homemade perfumes and fragrances
Handling
Safety
While not strongly toxic, α-pinene produces vapors that can cause respiratory problems and may act as a skin irritant as well. It should not be used near open flame due to its flammability.
Storage
Due to its volatility, α-pinene should be kept in airtight containers away from sources of open flames.
Disposal
α-pinene is commonly encountered in biological systems and does not pose a significant threat to the environment nor to humans. Given its low density and immiscibility with water, pouring it down a sink may not be a good course of action for disposal, with an outdoor trash can presenting a better option. However, it's best to have it soaked in a porous material, like sand or charcoal, to reduce any leaks on the ground or pavement.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Biologically-derived compounds
- Fragrant compounds
- Terpenes
- Hydrocarbons
- Liquids